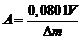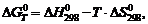Current Issue
Dispersion of Aluminum in Pulsed Discharges
Ramiz K Bayramov*
R.K. Bayramov, LLC. "Olympia" Novomoskovsk, st. Transportnaya, 26, 301650, Tula region, Russia
*Corresponding author: Ramiz K Bayramov, R.K. Bayramov, LLC. "Olympia" Novomoskovsk, st. Transportnaya, 26, 301650, Tula region, Russia, E-mail: [email protected]
Received Date: July 18, 2024
Publication Date: November 01, 2024
Citation: Bayramov RK. (2024). Dispersion of Aluminum in Pulsed Discharges. Catalysis Research. 4(1):19.
Copyright: Bayramov RK. © (2024).
ABSTRACT
The works available in the literature, including our own, were analyzed, on the basis of which this review article was prepared. A study of the process of aluminum dispersion in pulsed discharges in aqueous solutions has established that the main products obtained during the dispersion process are two aluminum hydroxides (bayerite and boehmite structures) and metal powder. It has been shown that bayerite aluminum hydroxide (AН) is obtained by dispersing the metal in pulsed discharges in distilled water, and boehmite AН - in weak solutions of nitric acid salts and some organic compounds. When the metal is dispersed in weak solutions of inorganic and organic acids, the process produces dispersed aluminum powder (crystallites have a spherical shape with a diameter of 20-60 nm). The influence of the nature and concentration of the components of the working solution, the temperature and exposure time of the pulp (a mixture of the working solution and dispersed metal), and the frequency of current pulses supplied to the electrodes on the formation of the product composition was studied. It has been shown that atomic oxygen (AO), formed during the thermal decomposition of the components of the working solution, plays an important role in the formation of the final products. It has been revealed that in the process of dispersing aluminum in weakly acidic solutions, the resulting thermal decomposition of AO forms a protective oxide film on the surface of the dispersed metal particles, protecting the dispersed metal from interaction with the environment. In this case, the result is a metal powder that does not have pyrophoric properties. A mechanism for obtaining aluminum dispersion products is proposed.
Keywords: Dispersion, Aluminum Hydroxide, Metal Powder, Atomic Oxygen, Pulsed Discharge
INTRODUCTION
As is known, the production of various substances or raw materials for different industries: the chemical and mechanical engineering industries, the automotive and aircraft industries, medicine, etc. is obtained mainly by the chemical method. Let's consider some examples of the production of aluminum hydroxide and metal powder [1-11]. In [1], aluminum hydroxide with a bayerite structure is proposed to be obtained by precipitation from an aluminum nitrate solution with an ammonia solution at room temperature. To obtain a coarse product, ammonia solution is used in constant excess, and precipitation is carried out in the presence of a seed - aluminum hydroxide obtained in the previous cycle. In [2], bayerite AН is obtained by precipitation from aqueous solutions of aluminum nitrate with ammonia under isothermal conditions at a constant pH value. The influence of the temperature of the reaction medium, the duration of the deposition process and aging on the chemical and phase composition of the resulting deposit was studied. It has been shown that bayerite is formed at low deposition temperatures (not higher than 60°C) and high pH values (10.0–11.0). The precipitation of bayerite should be additionally accompanied by aging of the formed precipitate in the mother solution while maintaining a constant temperature and pH, and the total precipitation time should be at least 2 hours. These results are confirmed by the data obtained in [3]. In work [4], boehmite aluminum hydroxide and hydrogen are obtained by preparing a suspension of powdered aluminum (aluminum powders with particle sizes of 60 microns are used) in water, creating in the reactor a pressure and temperature of saturated water vapor corresponding to the conditions for conducting an intense reaction of aluminum oxidation, spraying the suspension into reactor, removal of a mixture of water and hydrogen vapor from the reactor, as well as removal of boehmite from the reactor to the receiving device. When preparing a suspension of powdered aluminum in water, a catalyst - alkali metal hydroxide is introduced into it in an amount of no more than 0.1 M (M is the molar concentration), and after spraying the suspension until the boehmite is removed from the reactor, the suspension is kept for additional oxidation of aluminum and crystallization of boehmite. Aluminum hydroxide of pseudoboehmite structure in [5] is obtained by heat treatment of the original aluminum trihydroxide on a rotating surface heated to 100-7000C for 0.5-5 s, and then hydrothermal treatment of the resulting product is carried out at a temperature of 105–1700C for 2-48 hours in the presence of inorganic or organic substances. From the pseudoboehmite structure aluminum hydroxide obtained by this method, gamma aluminum oxide is obtained by heat treatment at a temperature of 500-7000C. The invention makes it possible to obtain highly dispersed aluminum hydroxide of pseudoboehmite structure and gamma aluminum oxide (g-Al2O3) with a high specific surface area. In [6], a new method for producing pseudoboehmite aluminum hydroxide was proposed, which consists of treating crystalline potassium alum with gaseous ammonia, leaching the product by ammonization with circulating solutions, separating the precipitate of hydrated aluminum oxide, and aging it in ammonia solutions at a pH of at least 10. The resulting pseudoboehmite is characterized by the specific surface area 260–360 m2/g with impurity content within the range, % (wt.): 0.015–0.025 Fe2O3; 0.03–0.05 K2O; 0.020–0.035 SiO2; 0.6–1.1 SO3. Loss on ignition - 32–40%. This creates real prerequisites for its use in the production of catalysts, adsorbents, and desiccants. In [7], a comparative analysis was carried out on the most common industrial technologies for the production of raw materials for catalyst carriers based on aluminum hydroxides. Modern developments in the field of obtaining the advantages and disadvantages of the described technologies are described.
In [8], metal powder is obtained by spraying molten aluminum with air, followed by sieving the resulting powder and grinding it in a gas environment containing nitrogen and oxygen. According to the invention, the gas formed during powder grinding, containing from 5 to 8% oxygen, is used as a gaseous medium when sieving powder. Simultaneous production of powders and powders is ensured without organizing special nitrogen production. The resulting aluminum powders and powders can be used in chemistry, energy, and electronics.
In industry, aluminum powders are produced [9] mainly by spraying molten metal using a protective (inert with respect to aluminum) gas environment - nitrogen under pressure from 6 to 25 atm. This produces a semi-finished product with a wide range of particles from 5 microns to 0.5 mm, which in practice is called atomizer. The resulting pulp is dispersed on a screen to obtain finished powders or sent for grinding to obtain aluminum powder. Significant disadvantages of the methods considered include the high consumption of reagents used, the formation of large quantities of contaminated wastewater, and the complexity of the technological process.
Despite the differences in the works [10,11] and not being close to the topic of the article, we will briefly consider them. In [10], a new nanoparticle collector (NC) was developed to improve the efficiency of bauxite desulfurization. It shows the optimal conditions for the synthesis of NC (with an average particle size of 252 nm), in which the ratio (wt.) of lignin, potassium butylxanthate and sodium dodecyl sulfate is respectively 1:20:2 at a temperature of 700C. It has been shown that in order to improve the characteristics of bauxite desulfurization (roasting, electrochemistry, flotation, etc.), it is necessary to know the preliminary desulfurization of bauxite [11]. It provides a detailed analysis of their mechanisms. Perhaps the use of this technology will lead to the production of higher quality raw materials (aluminum) used in the process of metal dispersion.
A number of studies have noted a significant influence on the formation of final products in the process of metal dispersion by the holding temperature of the pulp (a mixture of dispersed metal and working solution). It was revealed that in the working solutions used in the dispersion of aluminum (except for distilled water), with an increase in the pulp holding temperature, the pulp holding ti decreases, while in the resulting product there is an increase in the content of boehmite aluminum hydroxide to its maximum values (about 100%).
Interesting results were obtained in the works in [12-20]. In [12-14], bayerite aluminum hydroxide was obtained by dispersing aluminum in distilled water. In work [15], when dispersing aluminum with small additions of hydrogen peroxide (0.3 – 1.0% by volume), followed by boiling the mother solution (a mixture of working solution and dispersed metal) for ~ 6 hours, the formation of aluminum hydroxide with a boehmite structure was revealed. The formation of boehmite aluminum hydroxide during metal dispersion is also noted in [16,17]. Works [18-20] describe the production of aluminum powder by dispersing metal in pulsed discharges.
The purpose of this work is to determine the conditions and optimize the process of obtaining various products when dispersing aluminum in pulsed discharges.
EXPERIMENTAL PART
To increase the productivity of the process, in [12,13] they propose to space the electrodes at certain distances from each other, and fill the interelectrode gap with granules of dispersed metal. In this case, when current pulses are applied to the electrodes, spark discharges occur at the points of contact of the granules with each other and with the electrodes, and the metal is dispersed. Subsequently, this technique was used by many researchers in the development and creation of new reactors and in laboratory installations [21-27].
Laboratory setup
The experiments were carried out in a laboratory setup. The main components of the installation are a metal dispersion reactor and a current pulse generator. The reactor is a horizontally located cylinder made of organic glass, at the ends of which metal electrodes made of the dispersed metal are fixed. The interelectrode space is filled with granules of the desired metal. The working solution is pumped through the reactor at a certain speed. When current pulses are applied to the electrodes, spark discharges occur at the points of contact of the granules with each other and with the electrodes. In this case, the metal is dispersed [14]. The resulting pulp (a mixture of working solution and dispersed metal) is filtered. The filtrate is returned to the container of the original working solution, and the filtered precipitate is dried in air. As is known, a high temperature develops in the spark discharge zone, reaching ~ 10000ºС [28,29]. Under these conditions, both metal dispersion and thermal decomposition of the working solution occur to produce decomposition products. In the spark zone, the temperature reaches ~ 10000ºС, while at the same time in the reactor volume, by regulating the flow rate of the working solution, the temperature does not exceed 40°С. Due to the large temperature difference in the discharge zone and in the reactor volume, rapid cooling of the dispersed metal occurs with the formation of particles of an ultradisperse structure.
Determination of aluminum content in the resulting products
The aluminum content in erosion products was determined according to the method described in [30]. A sample of the dried product is loaded into a conical flask. Then, from a graduated cylinder filled with an alkali solution, an alkali solution is introduced into the flask. In this case, aluminum reacts with the alkali, releasing heat and producing hydrogen, which is fed into a calibrated gas burette. The volume of hydrogen released was brought to normal conditions. The metallic aluminum content is calculated using the formula:
where A is aluminum content, wt.%; V is the volume of hydrogen released,
reduced to normal conditions, ml; ∆m; – difference in mass of a sample before and after interaction with an alkali solution, g.
Determination of the phase composition of metal dispersion products in pulsed discharges
The phase composition of the resulting sediment was determined using a diffractometer (DRON-3) with copper radiation in continuous mode and recording a diffraction pattern. When analyzing the phase composition of the samples, the values of interplanar distances of the crystal lattice were marked on the diffraction pattern using the ASTM data catalog and other literature sources [31]. Interplanar distances are indicated for diffraction lines: bayerite - 1.33; 1.39; 1.44;1.55;1.60; 1.72;2.23;2.47;3.23;4.40;4.80, pseudoboehmite – 1.42;1.85;2.34;3.20;6.30 and aluminum powder – 1 .22;1.40;2.02;2.33.
Electron microscopic studies of products obtained during the dispersion process
The shape and size of dispersed metal particles were determined using a transmission electron microscope (EVM-100LM). The resulting metal powder was preliminarily suspended in isopropyl alcohol, then the suspension was dispersed on an ultrasonic dispersant (UZDN-1) and then applied to an organic substrate film. Photographs of the samples were carried out at different magnifications. From the resulting images, the shape and size of the crystallites under study were determined.
Development of prototypes and industrial samples of current pulse generators and reactors
The interest aroused in this method attracted the attention of a number of researchers to participate in the development of designs of more powerful current pulse generators, high-performance reactors for the creation of experimental and pilot industrial installations for metal dispersion [32-34]. Installation of such installations will lead to a sharp increase in process productivity with the production of tens and hundreds of tons of product.
RESULTS AND THEIR DISCUSSION
Products obtained in the process of aluminum dispersion
Figure 1 shows a diffraction pattern of bayerite aluminum hydroxide (HA), obtained by dispersing aluminum in distilled water [14,15].
Figure 1. Diffraction pattern of bayerite AН obtained by dispersing aluminum in pulsed discharges in distilled water.
The holding temperature of the resulting pulp is +200C, the holding time of the pulp is 20 hours
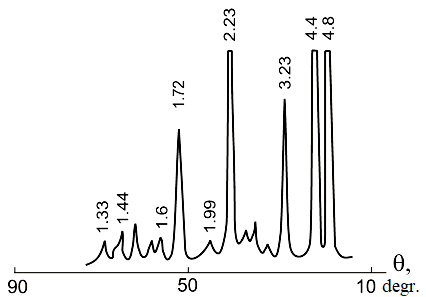
The data obtained are consistent with the results described in [13], which proposed a mechanism for the formation of bayerite HA. It will be discussed in detail a little later when discussing the mechanism for obtaining products when dispersing aluminum in pulsed discharges. Carrying out the process of dispersing the metal in weak solutions of acid salts (for example, ammonium nitrate) leads to the formation of boehmite aluminum hydroxide (AlOOH), the diffraction pattern of which is shown in Figure 2 [16,17].
Figure 2. Diffraction pattern of boehmite AН obtained by dispersing aluminum in pulsed discharges in a 0.12 M solution of ammonium nitrate.
Pulp holding temperature +900C, pulp holding time 9 hours.
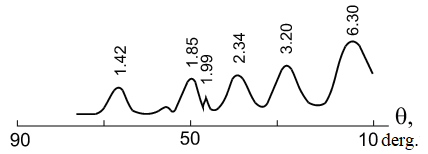
The process of dispersing aluminum in weak acid solutions (for example, nitric acid) contributes to the formation of metal powder (Figure 3) [18-20].
Figure 3. Diffraction pattern of aluminum powder obtained by dispersing the metal in pulsed discharges in a 0.10 M nitric acid solution.
Pulp holding temperature +200C, pulp holding time 6 hours.
It has been shown that the formation of final products during the dispersion process is significantly influenced by the nature and concentration of the working solution, the temperature and holding time of the pulp (a mixture of dispersed metal and working solution).
The influence of pulp exposure time during the production of pure boehmite HA in the process of dispersing aluminum in the same solution (for example, NH4NO3) of different concentrations has been established. The pulp holding time decreases with increasing salt concentration (11 hours in a 0.1 M NH4NO3 solution to 8 hours in 0.18 M NH4NO3). This is probably due to different amounts of atomic oxygen produced in the discharge zone, and, as a consequence, to different proportions of metal particles covered with a hydrated oxide film.
A similar reduction in the holding time of the pulp when obtaining pure boehmite HA also occurs in the process of dispersing the metal in solutions containing components with different amounts of oxygen in the molecule. In this case, this circumstance, also as noted above, is due to different amounts of atomic oxygen formed during the dispersion process, and, as a consequence, to different proportions of metal particles coated with a hydrated oxide film.
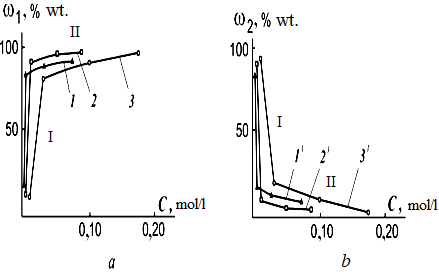
Data on the influence of various acids containing oxygen (nitric, sulfuric acids) and not having it in their composition (hydrochloric acid) on the composition of products obtained by dispersing aluminum are presented in Figure 4 [19].
Figure 4. Dependence of the content (w1, a) of aluminum powder (1−3) and the total content (w2, b) of aluminum hydroxides in the metal dispersion products on the concеntration of acids (C) in the working solution. Working solution: 1−1¢ —HCI solution; 2−2¢—HNO3 solution; 3−3¢ – H2SO4 solution.
Holding time at a temperature of 20°C is 4 hours.
It follows from the figure that two sections can be distinguished on the w−C curves: section I in the concentration range of 0.007...0.03 моль×л–1; section II – 0.01…0.18 моль×л–1 . In the 1st section, with an increase in the concentration of acid solutions, there is a sharp increase in the content of metal powder and a decrease in the total content of aluminum hydroxides in the resulting product. Moreover, the stronger the acid, the more sharply the content of metal powder in the product increases. A further increase in the concentration of acid solutions has little effect on the content of metal powder in the product (section II). The observed nature of the change in the w−C curves is obviously associated with a different mechanism of the processes of formation of the oxide film. As noted, in the spark discharge zone, there is enough energy for the formation of oxygen from oxygen-containing acid residues. The resulting oxygen can participate in the oxidation of metal particles to form an oxide film on their surface. However, a sharp increase in the content of metal powder in the resulting product is observed during the dispersion process in section I and when one of the acids (hydrochloric) does not have an oxygen-containing acid residue. This may indicate that oxygen from the oxygen-containing acid residue, as well as oxygen from water molecules, does not participate in the oxidation reaction of dispersed metal particles in section I. It is known that in dilute aqueous solutions of acids, hydrogen ions are in hydrated form in the form of hydronium ion (H3O+). Probably, the source of oxygen in this case is H3O+, which, entering the spark discharge zone, thermally decomposes with the formation of oxygen. This can explain the sharp increase in the content of metal powder in section I of the w−C curve with an increase in the concentration of acid solutions, including hydrochloric acid. In section II of the w−C curve, the content of metal powder changes slowly with increasing acid concentration. Moreover, the slowest growth occurs when hydrochloric acid is used in the process. Most likely, this is due to the fact that in section II, in the oxidation reaction of dispersed aluminum, together with oxygen formed during the thermal decomposition of hydronium ions, oxygen, obtained during the thermal decomposition of the oxygen-containing acidic residue, takes part. Therefore, in this concentration range, metal oxidation occurs most slowly and incompletely when hydrochloric acid is used in the process. The addition of acids to the working solution ensures that during the dispersion of the metal, a protective oxide film is formed on the surface of the dispersed aluminum particles, which prevents the interaction of the dispersed metal with water. The observed phenomenon is practically independent of the type of acid, which does not agree with traditional ideas about the effect of acids on aluminum under conditions close to normal. A protective oxide film on aluminum, as is known, is formed in the cold in a concentrated solution of nitric acid [35], the formation of a protective oxide film in solutions of hydrochloric acid under normal conditions is not indicated in the literature. Under conditions of aluminum dispersion in hydrochloric acid solutions, at the initial moment, even more intense formation of a protective oxide film occurs than in the presence of nitric and less strong acids.
Analysis of the experimental data obtained showed that the reason for the formation of different products during electric spark dispersion of aluminum should be sought in the processes occurring in the spark discharge zone. In this case, it becomes necessary to determine the intermediate product formed in the discharge zone. However, due to the high temperature in the spark discharge zone (~10 000 °С) [28, 29] analysis of the intermediate product is not possible, and therefore a simplified procedure was developed, which is as follows. Pulp samples were taken from the pulp collection container, which, without being kept at temperature, were filtered. The filtrate was returned to the cycle, and the filtered precipitate was dried in air and then its phase composition was determined.
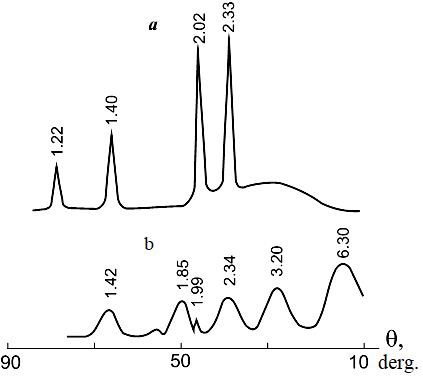
The diffraction pattern of the product obtained in this way from the pulp formed by electric spark dispersion of aluminum in a solution of ammonium nitrate is shown in Figure 5.a,b.
Figure 5. Diffraction pattern of the products of electric spark dispersion of aluminum in a 0.1 M solution of ammonium nitrate. Pulp holding temperature + 90С; pulp holding time: a – 0 hours, b – 9 hours.
It was found that the resulting product consists mainly of dispersed metallic metal particles and a small amount of amorphous aluminum hydroxide. The amorphous state of the metal hydroxide corresponds to a halo in the Bragg angle region of 20...40 degrees. When the same pulp is kept at a temperature of 90°C for 9 hours, a product is obtained that is boehmite AН (Figure 5b), i.e. formed as a result of the interaction of the intermediate product with water in the reactor volume.
The production of various modifications of AН (bayerite, boehmite) in the process of metal dispersion largely depends on the amount of oxygen formed in the process. When dispersing aluminum in distilled water, due to the production of a small amount of oxygen in the discharge zone during thermal decomposition of the working solution, a small amount of metal particles coated with a hydrated oxide film is formed. As a result, when the resulting pulp is kept at a temperature of 20°C, metal particles in the volume and small amounts of particles coated with a hydrated oxide film interact with water to produce bayerite AН in the process. In the case when, during the dispersion process in pulsed discharges, significant amounts of oxygen are formed, which, reacting with dispersed particles, contribute to an increase in the proportion of dispersed metal with a hydrated oxide film, and as a consequence, in the volume of the reactor, when interacting with water, leads to the formation of boehmite AН. This assumption is consistent with the experimental data obtained.
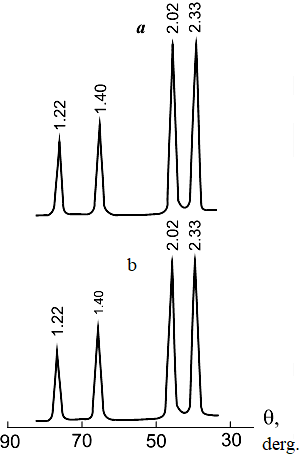
Using a simplified procedure, it is also possible to determine the intermediate product formed in the spark discharge zone and when dispersing aluminum in solution in weakly acidic solutions (for example, nitric acid, Figure 6 a, b.).
Figure 6. Diffraction patterns of aluminum erosion products in a 0.1 M nitric acid solution. Pulp holding temperature + 20°С; pulp holding time: a – 0 h; b − 4 hours.
The figure shows that the product obtained from a pulp that has not been subjected to exposure at temperature (Figure 6 a) is practically no different from the product obtained from the same pulp, but subjected to exposure (Figure 6 b), which indicates the formation of a protective oxide film on the surface of the dispersed metal, preventing interaction of the metal with water. As a result, the dispersion process produces a metal powder that does not have pyrophoric properties.
To expand the scope of application of additives in the working solution, research into the process of dispersing aluminum with other additives was continued. The results obtained are presented in Table 1.
Table 1. The influence of components introduced into the working solution on the composition of products obtained during the dispersion of aluminium in pulsed discharges.
|
The component introduced into the working solution, g |
Concentra-tion (C), mol / l
|
Pulp holding tempera-ture, 0 C |
Exposure time ̀ (t), hours |
DЕ, кJ/mol |
Composition of products, % by weight |
|||
|
Boehmite aluminum hydroxide |
Bayerite aluminum hydrox-cide |
Alumi- num powder |
||||||
|
Distilled water |
- |
25 |
20 |
910 |
9 |
100 |
- |
|
|
Glycerol |
0,011 0,032 0,125 |
90 90 90 |
9 9 9 |
670 |
70 85 100 |
30
15 0 |
- - - |
|
|
Sucrose |
0,007 0,034 0,450 |
80 80 80 |
8 8 8 |
650 |
80 100 100 |
20 0 0 |
- - - |
|
|
Oxalic acid |
0,055 |
25 |
5 |
640 |
ΣАH = 8 |
92 |
||
|
Nitric acid |
0,040 |
25 |
4 |
350 |
ΣАH = 9 |
99 |
||
|
Hydrochloric acid |
0,34 |
25 |
2
|
-
|
ΣАH = 9 |
91
|
||
To clarify the reasons for the growth of boehmite AH, the values of the energy of formation of atomic oxygen ((DЕ) during the thermal decomposition of the working solutions used in the process were determined using a quantum chemical method. It follows from the table that in all working solutions used in the dispersion process; (DЕ) is less than in water. At the same time, the atomization energy was the lowest for nitric acid. The data obtained suggest that in the process of metal dispersion, boehmite aluminum hydroxide is formed as a result of an increase in the amount of oxygen in the discharge zone and, as a consequence, the proportion of dispersed metal with a surface covered with a film of hydrated oxide. which reacts with water in bulk.
The addition of acids to the working solution ensures the formation of a protective oxide film on the surface of dispersed aluminum particles when dispersing the metal, preventing the interaction of the dispersed metal with water. The observed phenomenon is practically independent of the type of acid, which does not agree with traditional ideas about the effect of acids on aluminum under conditions close to normal. A protective oxide film on aluminum is known to form in the cold in a concentrated solution of nitric acid [35], the formation of a protective oxide film in hydrochloric acid solutions under normal conditions is not indicated in the literature. Under conditions of aluminum dispersion in hydrochloric acid solutions, at the initial moment, even more intense formation of a protective oxide film occurs than in the presence of nitric and less strong acids.
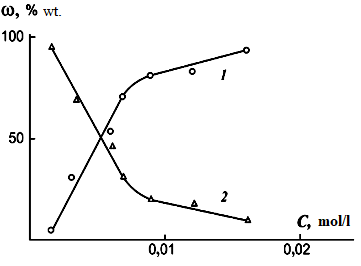
Calculations have shown that the reaction of the formation of atomic oxygen from hydronium ion requires ~4.5 times less energy (DЕ) = 210 kJ•mol–1) than from water. The assumption that the oxidation of dispersed metal involves oxygen formed during the thermal decomposition of hydronium ions was confirmed by the results obtained by dispersing aluminum in a solution of ammonium nitrate with the gradual introduction of nitric acid into it (Figure 7).
Figure 7. Dependence of the content (w) of aluminum powder (1) and the total content of aluminum hydroxides (2) in the metal dispersion products on the concentration of nitric acid (C) added to a 0.025 M working solution of ammonium nitrate. Pulp exposure at a temperature of +200С, pulp exposure time - 4 hours.
The nature of the change in the content of metal powder in the dispersion product with the introduction of nitric acid into the working solution indicates an increase in the amount of oxygen generated in the process in the spark discharge zone, leading to the growth of metal particles with a surface covered with a protective oxide film. As a result, the content of metal powder in the resulting product increases. Since the working solution contains substances with the same anion - nitrate ion, the increase in the content of metal powder is caused by an increase in the concentration of hydronium ions.
It has been established that the formation of aluminum metal powder also occurs when aluminum is dispersed in solutions of one (or two) substituted salts of polybasic carboxylic acids. Table 2 shows data on the effect of the concentration of salts of polybasic carboxylic acids (ammonium tartrate and citrate) introduced into the working solution.
Table 2. Effect of salts of polybasic carboxylic acids (ammonium tartrate and citrate) introduced into the working solution on the composition of the products formed during the dispersion of aluminum.
|
Introduced into solution salt. |
Salt concentration, mol-1 |
Amount of oxygen in salt |
Composition of products, % wt. |
|
|
Metal powder |
SGA |
|||
|
Monoammonium Tartrate. |
0,033 |
6 |
90 |
10 |
|
0,054 |
6 |
93 |
7 |
|
|
0,075 |
6 |
96 |
4 |
|
|
Disubstituted ammonium citrate. |
0,029 |
7 |
94 |
6 |
|
0,048 |
7 |
96 |
4 |
|
|
0,067 |
7 |
98 |
2 |
|
Pulp holding temperature -200C, pulp holding time -4 hours.
SGA is the total content of all forms of aluminum hydroxide.
The mechanism of formation of aluminium dispersion products in pulsed discharges
Metal powders obtained under rapid cooling conditions have a number of technological advantages compared to conventional powders [36,37]. For example, all sintering operations of products made from these powders occur at lower temperatures and with shorter exposure times. Products compacted from such powders have increased strength, ductility and corrosion resistance. Unlike highly dispersed metal powders obtained by other methods, metal powders formed during the dispersion process do not have pyrophoric properties, which is due to the formation of a protective oxide film on their surface. This creates favorable conditions for storing and transporting such powders.
During the dispersion process in the spark discharge zone, along with the dispersion of the metal, thermal decomposition of the working solution occurs with the formation of decomposition products, including atomic oxygen, which, reacting with metal particles, forms a hydrated oxide (or protective oxide) film on their surface. To determine the reliability of this assumption, the thermodynamic possibilities for the occurrence of oxidation reactions of dispersed metal particles were determined. Changes in the standard Gibbs energy for reactions were calculated using the equation [38]:
where is the change in standard enthalpy, kilojoule. Mol/1; T – temperature, K; − change in standard entropy, kilojoule. mol-1.
In the calculations, data taken from work [39] were used. The calculation results are presented in Table 3.
Table 3. Changes in the standard Gibbs energy for reactions occurring during thermal decomposition of working solutions and the interaction of dispersed metal particles with oxygen.
|
Reaction |
|
|
1. Н2О(L) = Н2(G) + О(G) |
+468,5 |
|
2. Н2О2(L) = Н2О(G) + О(G) |
+115,0 |
|
3. Н3О+ = 1,5 Н2(G) + О(G) |
−439,3 |
|
4. 2Al(Cr) + 3O(G) = Al2O3(Cr) |
−2278,1 |
|
5. 2Al(Cr) + 1,5O2(G) = Al2O3(Cr) |
−1582,7 |
From Table 3 it is clear that under standard conditions the change in the Gibbs energy for reactions 1 and 2 has a positive value and only for reaction 3 it has a negative value. Thus, in the examples considered, only reaction 3 is thermodynamically possible. In the spark discharge zone, as noted earlier, the temperature reaches ~10,000 °C. In this regard, it is of interest to determine the change in the Gibbs energy for the reactions presented in the table under numbers 4 and 5. It follows from the table that the 4th and 5th reactions are thermodynamically possible, since in both cases the change in the Gibbs energy is negative. However, the 4th reaction is most likely to occur, for which DG has a smaller value compared to the fifth, i.e. interaction of dispersed metal particles can only occur with atomic oxygen (AO). This assumption is logical, since with the probability of the 5th reaction occurring, there is a need for preliminary dimerization of oxygen atoms into molecules, which is impossible.
In [13] it was noted that the process of dispersing aluminum in water produces bayerite aluminum hydroxide. The authors proposed a mechanism for its formation, which consists in the interaction of dispersed metal particles with OH radicals obtained during the dispersion process. However, this mechanism is not entirely substantiated; it cannot explain the production of less hydrated products – boehmite AH and metal powder – during the dispersion process.
To determine the shape and size of crystallites formed during the dispersion of aluminum in pulsed discharges, electron microscopic studies were carried out. Figure 8 shows photographs of samples obtained during the dispersion of metal in different working solutions, from which the shape and size of crystallites can be determined.
Figure 8. Electron microscopy images of bayerite AH (a), boehmite AH (b) aluminum powder (c) – products of aluminum dispersion in pulsed discharges.
Bayeritic AH (a) – working solution – distilled water,
Boehmite AH (b) - working solution - 0.14 M ammonium nitrate solution,
Aluminum powder (c) – 0.12M nitric acid solution.
Pulp holding time at a temperature of +200 C, a - 20 hours,
+900С, b – 9 hours,
+200C, c – 4 hours.
Aluminum powder crystallites have a spherical shape with dimensions of 20-60 nm, boehmite crystallites are needles with a width of ~ 20 and a length of 130 nm. Bayerite crystallites have the shape of a parallelepiped with a width of 40-70 and a length of 120-140 nm.
In all three cases, coagulation of crystallites into secondary aggregates occurs over time [35]. Only a small part (25-35 wt.%) of the metal powder remains uncoagulated.
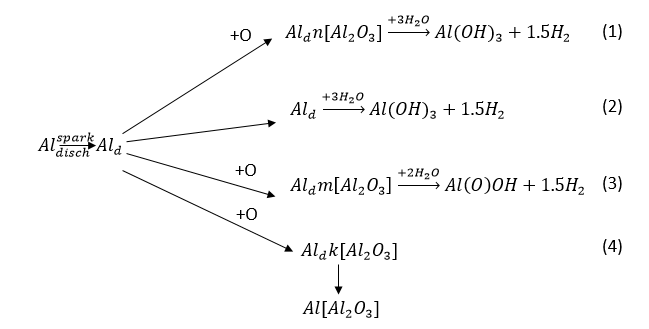
Analysis of the literature and obtained experimental data allows us to present the scheme of product formation during the dispersion of aluminum in pulsed discharges as follows (Figure 9).
Figure 9. Scheme of product formation in the during dispersion process of aluminum in aqueous solutions: Al – compact aluminum; Ald – dispersed aluminum; [Al2O3] AIdk– dispersed aluminum with a surface covered with a protective oxide film; Aldm[Al2O3] – aluminum coated with a hydrated oxide film (m); Aldn[Al2O3] – aluminum coated with a hydrated oxide film (n), with m >> n; Al(Al2O3) – aluminum powder; Al(O)OH – boehmite; Al(OH)3 – bayerite; O – atomic oxygen.
Small amounts of formed AO (less than 6-8% wt.) lead to the formation of bayerite aluminum hydroxide (AН) (reaction 1), and this is due to the fact that only a small part of the metal particles are oxidized by AO to form a hydrated oxide film on the surface (reaction 1). The main part of AН bayerite is formed as a result of the direct interaction of reactive aluminum particles with water (reaction 2).
An increase in the amount of atomic oxygen when dispersing a metal in dilute solutions of salts of oxygen-containing acids and some organic compounds (glycerol, sucrose) leads to complete oxidation of metal particles with the formation of an intermediate product (dispersed metal particles with a surface covered with a film of hydrated oxide). Next, the intermediate product in the reactor reacts with water to form boehmite AH (reaction 3). When the process produces AO in an amount sufficient to oxidize metal particles with the formation of a protective oxide film on their surface, the process produces aluminum powder that does not have pyrophoric properties (reaction 4).
CONCLUSION
Analysis of literary and experimental data allows us to present the process of aluminum dispersion in pulsed discharges with the production of various products as follows. It has been revealed that atomic oxygen (AO), formed in the discharge zone, plays an important role in the formation of various compounds. It has been shown that in small quantities AO oxidizes a small portion of dispersed metal particles to form a hydrated oxide film on their surface, which reacts with water in the reactor volume to form bayerite aluminum hydroxide (AН). The bulk of bayerite AН is obtained as a result of the interaction of reactive aluminum particles with water. An increase in the amount of AO leads to complete oxidation of the resulting metal particles with the formation of a hydrated oxide film on the surface, which reacts with water to form boehmite HA. It has been established that in the process of dispersing a metal in dilute solutions of inorganic acids and some organic compounds, a large amount of AO is formed, which oxidizes the metal particles with the formation of a protective oxide film on their surface, protecting the metal from interaction. with the environment. As a result, the dispersion process produces a metal powder that does not have pyrophoric properties.
REFERENCES
- Patent SU 236438. Bulgakova YuL, Vinnikova TS, Dzisko VA. Method for producing aluminium hydroxide. Published 03.10.2000.
- Tagandurdyeva N, Naraev VN, Postnov AYu, Maltseva NV. (2020). Preparation of aluminum hydroxide - bayerite by precipitation method. News of St. Petersburg State Technical University (TU). 53:17-22.
- Busca G. (2014). Structural, Surface and Catalytic Properties of Aluminas. Advances in Catalysis. 57:319-404.
- Patent RU 2363659. Bersh AV, Ivanov YuL, Mazalov YuA, et al. Method for producing boehmite and hydrogen. Published 08.10.2009.
- Patent RU 2234460. Zolotarev IA, Ivanova AS, Karasyuk IV, et al. A method for producing aluminum hydroxide of pseudoboehmite structure and gamma aluminium oxide based on it. Published 20. 08.2004.
- Matveev VA, Zakharov VI, Mayorov DV, et al. (2012). Study of the production of aluminum hydroxide of pseudoboehmite structure. Non-ferrous metals. 4:46-49.
- Knyazev AV, Lavrov BA. Modern technologies for the production of aluminum hydroxide raw materials for catalyst carriers. News of St. Petersburg State Technical University (TU). 2021. N. 59. Chemistry and chemical technology - chemistry and technology of inorganic substances. pp.37-46.
- Patent RU 2204462. Galanov AI, Gopienko VG, Volkov IV, et al. Method for producing aluminum powders and powders. Published. 20.05.2003.
- Gopienko VG. (1980). Production and use of aluminum powders and dusts. M: Metallurgy. 67p.
- Cheng G, Jianjiang, Su H, Zhang Z. (2022). New collector for flotation desulfurization of high-sulfur bauxite. Proceedings of the Non-Ferrous Metals Society of China. 32:86-100.
- Cheng G, Li YL, Jang M. (2022). Progress in research into high-sulfur bauxite desulfurization technology. Proceedings of the Non-Ferrous Metals Society of China. 32:3374-3387.
- USP 3355279. Method and apparatus for manufacturing micro fine powder. Wataru Ishibashi Field: 05.18.65.Date of Pat. 28.11.67.Official Gazette. 844(4):1438-1439.
- Ishibashi W. (1977). The Reaction between Powder and Liguid Medium of Spark Disharge Poind. J Jap Soc Powder and Powder Met. 24:113-117.
- Bayramov RK, Vedernikova NR, Ermakov AI. (2001). Electrospark dispersion of aluminum and its subsequent hydration. Journal of Applied Chemistry. 74(10):1703-1706.
- Patent SU 592753. Tsoi AD, Petrenko BYa, Asanov UA, et al. Method for producing aluminum hydroxide. Published 15. 02.78.
- Patent SU 919278. Bayramov RK, Sabanin AV, Gorozhankin EV, et al. Method for producing pseudoboehmite aluminum hydroxide. Published 12.07.81.
- Bayramov RK, Ermakov AI, Vedernikova NR. (2001). The influence of some organic compounds on the composition of products of electrospark dispersion of aluminum. Journal of Applied Chemistry. 74(10):1708-1710.
- Bayramov RK. (2009). Formation of aluminum powder during electric spark dispersion of metal in aqueous solutions. Non-ferrous metals. 10:69-71.
- Bayramov RK, Ermakov AI, Vedernikova NR. (2002). Behavior of aluminum during its electric spark dispersion in aqueous solutions of certain acids. Journal of Applied Chemistry. 75(3):419-421.
- Bayramov RK. (2003). Behavior of metal particles formed during electric spark dispersion in aqueous solutions of acids. Journal of Applied Chemistry. 76(7):1067-1070.
- Patent SU 12514.31. Kazekin VI, Gorozhankin EV, Frolov VF, et al. Installation for electroerosive production of metal powders. Published 15.04.1986.
- Fominsky LP. (1983). Installation for producing highly dispersed powders. Electronic technology. Ser Materials. 3:49-52.
- Patent SU 1077743. Kazekin VI, Savelyev IV, Gorozhankin EV, et al. Device for electroerosive dispersion of metals. Published 11.08.83.
- Patent SU 1039648. Yukhtin VA, Teplyakova RV, et al. Device for electroerosive dispersion of metals. Published 09.07.83.
- Patent SU 1463392. Kazekin VI, Cherepanov VP. Device for electroerosive dispersion of metals. Published 03.07.89.
- Patent SU 1381042. Karvovsky VB, Krapivina SA, Gorozhankin EV, et al. Device for electroerosive dispersion of metals. Published 15.11.87.
- Patent SU 997998. Fominsky LP. Method of electroerosive dispersion and device for its implementation. Published 23.02.88.
- Drabkina SI. (1951). On the theory of development of a spark discharge channel. Journal of Experimental and Theoretical Physics. 21:473-483.
- Arenkov AB. (1962). Fundamentals of electrophysical methods of materials processing. L. Mechanical Engineering. 372 p.
- Tolmachev IP. (1938). Production of aluminum powder, aluminum powder, paste. M. GONTI, 80p.
- Markin LI. (1961). Handbook on X-ray diffraction analysis of polycrystals. M: State Publishing House of Physical and Mathematical Literature. 562 p.
- Patent SU 1197066. Millikan AN, Shcherba AA, Muratov VA, et al. Pulse generator for electroerosive dispersion of conductive materials. Published 07.12.85.
- Patent RU 2200589. Tarasov VI, Kozyaruk OI, Fominsky LP. Device for electroerosive dispersion of metals. Published 11/15/93.
- Patent RU 2255837. Magnitsky YYu, Kozyaruk OI, Zhuravel SN. Device for electroerosive dispersion of metals. Published 10.07.2005.
- Glinka NL. (2000). General chemistry/Ed. A.I. Ermakova. M: Integral-Press. 728 p.
- Maringer RE. (1980). Production and processing of rapidly quenched aluminum powders. SAMPE Quarterly. 11(4):30-34.
- Danherty TS. (1964). Aluminum Sheet from Finely Divided Particles. Journal of Metals. pp. 827-833.
- Eremin EN. (1974). Fundamentals of chemical thermodynamics. M: Higher school. 342 p.
- Brief reference book of physical and chemical quantities / Ed. A.A. Ravdelya, A.M. Ponomareva. L.: Chemistry. 1983. 231 p.
 Abstract
Abstract  PDF
PDF