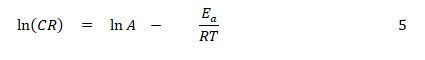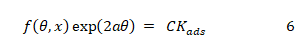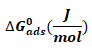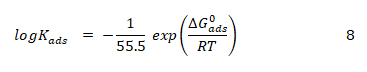Past Issues
Corrosion Inhibition of Mild Steel and Aluminum in Hydrochloric Acid Solutions by Gum Exudates from Erythrophleum Africanum
Ali Sunday Clement1, Shehu Garba1, Paul Ocheje Ameh2*
1Department of Chemistry, Faculty of Science, Nigerian Defence Academy, Kaduna, Nigeria
2Department of Chemistry, Nigeria Police Academy, Wudil Kano State, Nigeria
*Corresponding Author: Paul Ocheje Ameh, Department of Chemistry, Faculty of Science, Nigerian Defence Academy, Kaduna, Nigeria; Email: [email protected]
Received Date: March 31, 2023
Publication Date: April 24, 2023
Citation: Clement AS. (2023). Corrosion Inhibition of Mild Steel and Aluminum in Hydrochloric Acid Solutions by Gum Exudates from Erythrophleum Africanum. Catalysis Research. 3(1):10.
Copyright: Clement AS. © (2023).
ABSTRACT
The corrosion inhibition potentials of gum exudates extracted from Erthrophleum Africanum on mild steel and aluminium in acidic media were studied using gravimetric, Fourier transform infrared (FTIR) and scanning electron microscopy (SEM) and potentiodynamic methods. The results range for gravimetric showed (30.30-80.0 %) and potentiodynamic polarization (66.50-86.74 %) which shows that the inhibition efficiency of the inhibitor increases with increase in concentration of gum exudate but decreases with increasing temperature. The addition of the gum exudate affects both the cathodic and anodic partial reaction, thereby reducing the cathodic and anodic current densities and the corresponding corrosion densities, which makes the gum act as a mixed type of inhibitor. The inhibitor was found to be an adsorption inhibitor that best fits the Temkim adsorption model; because the data have high degrees of linearity and the parameters were positive. The activation energy values ranges from 10 - 20 J/mol which indicates that the adsorption of the inhibitors favors the mechanism of physical adsorption, free energy of absorption ranges from -10.51 to -11.69 J/mol which indicate that the reaction is spontaneous and it’s an exothermic reaction. FTIR spectra of the inhibitors and the corrosion products of mild steel and Aluminum (in the presence of the inhibitors) revealed that there were shifts in frequencies of adsorption (821,925,1620,1730,1990) suggesting that some functional groups were used in adsorption and some new bonds were formed. (CH Rocking, = CH ‘oop’, C=O symmetric stretch) Examinations of the morphology of the mild steel and Aluminum (using SEM) before and after inhibition revealed the formation of protective coverage over the surface of the metal
Keywords: Corrosion inhibition, Adsorption isotherm, Erthrophleum africanum, Natural polymers
INTRODUCTION
Corrosion causes gradual decay and deterioration in metals when exposed to the action of fluids in industrial processes. Among the effective methods of combating corrosion, the use of inhibitors is one of the best and widely used methods for steels in the oil and gas industry and has proven to be very successful (Ameh 2020) [1]. By definition, an inhibitor is a chemical compound which when added in a small amount to the corrosive environment alters the cathodic and or anodic reaction and subsequently reduces the corrosion rate (Hosseini et al., 2007) [2].
The safety and environmental issues in the use of corrosion inhibitors have always been of global concern. Safety is an important consideration in selecting any material in order to meet stringent requirements for engineering application among other properties such as mechanical, relative weight, reliability, cost and availability. Notwithstanding the importance of the environmental impact of corrosion as a problem which has been a motivation over the years for researchers to 2 focus on the use of corrosion inhibitors from the eco-friendliness, biodegradability and renewability point of view. According to Eddy and Ebenso (2008) [3], green corrosion inhibitors are obtained from plant and animals. They are non-toxic and do not contain heavy metals or other toxic substances. (Eddy et al., 2009b) [4] further stated that green inhibitors are relatively cheap and can be easily produced and purified, in addition, they are biodegradable. Most green corrosion inhibitors are extract of plants and animal products. The use of green inhibitors is preferred because they are eco-friendly. The use of gums as corrosion inhibitors has attracted considerable attention recently. (Ameh and Eddy, 2020) [1]. Gums are classes of high molecular weight polymeric compounds composed mainly of -CHO and N which are capable of possessing colloidal properties in an appropriate solvent, or swelling agent at low dry weight (Ameh et al 2012) [5].
Gum exudates from Erythrophleum africanum are of interest, because of their availability, more importantly relatively less expensive and environmentally friendly amidst other plants of biodegradable and renewable sources. Erythrophleum africanum is a small tree with a spreading, fairly dense crown; it can grow up to 15m tall; the straight, cylindrical bole can be unbranched for 10m and up to 120 cm in diameter. Most of the compounds extracted from these plants are been used in traditional medicinal applications such as aromatic spices, medical plants and herbs (malaria therapy and pharmaceutical drug development). However, the use of their gum exudate as corrosion inhibitors to best of my knowledge has not been tested.
This work covers the inhibition efficiency of Erythrophleum africanum gum on mild steel and aluminum in solutions of HCl using weight loss (gravimetric method), potentiodynamic polarization method, Fourier transform infrared (FTIR) and scanning electron microscopy.
MATERIAL AND METHODS
Collection of Gums
Crude Erythrophleum africanum gums (3 kg) was obtained as dried exudates from their parent trees grown at Taura Local Government Area of Jigawa State. The gum was collected from different EA plant species by tapping. An axe was used to break the outer bark of the tree. The cut was extended upward and downward to a significant depth and the gum formed was collected. The gum droplet collected was exposed to the atmosphere to dry and hardened (Eddy, 2011) [6].
Sample Preparation
Modified method of (Ameh et al., 2012) [7] was adopted for the treatment of the gum. Different samples of the gum were hydrated in chloroform for three days with intermittent stirring to ensure complete dissolution and then, strained through 75 μm sieve to obtain particulate free slurry which was allowed to sediment. Therefore, the gum was precipitated from the slurry using absolute ethanol filtered and washed with di-ethyl ether to defat the gum. The precipitate was dried in the oven at 40oC for 48 hours. The dried flakes were then pulverized using a blender and stored in an air tight container.
Corrosion Studies
Corrosion measurements were undertaken using standard procedure such as the following, the mild steel and aluminum sheets were mechanically press-cut into different coupons each of dimension, 3 x 3 x 0.14 cm. Each coupon used is degreased by washing with ethanol, cleaned with acetone and allowed to dry in air before preservation in a desiccator.
Gravimetric Method (Weight Loss)
The weight loss experiments were performed at as showed in the experimental set up given in Plate 1
Plate 1: Experimental setup for weight loss studies
In the experiment, weighed mild steel and aluminum coupons were completely immersed in 250 ml of the test solution (HCl + Distill water and NaOH + Distill water) in an open beaker. The beaker was covered with aluminum foil and inserted into a water bath maintained at 303 K (30 0C). After every 24 hours, the corrosion product was removed and washed (withdrawn from the test solution) in a solution containing 50 % NaOH and 100 g of zinc dust. The washed coupon was rinsed in acetone and dried in the air before re-weighing. The experiment was repeated at 333 K (60 0C), in each case the difference in weight over a period of 168 hours was taken as the total weight loss. From the average weight loss (mean of 3 replicate analysis) result, inhibition efficiency (I %) of the inhibitor, the degree of surface coverage (ɵ) and the collision rate of metal (mild steel and aluminum) (CR) were calculated using equation 1,2 & 3 respectively (Eddy, 2010) [8];
W = weight loss = W2 – W1
D = density of the metal in g/ cm3
Where D = mass / volume of the substance
A = total surface area in m
T = exposure time in hours, in various media
Where CRo and CRi are the corrosion rates in the absence and presence of various concentrations of EA gum exudate respectively
%IE = percentage inhibition efficiency
ϴ = degree of surface coverage
Electrochemical studies
This was conducted using a PARC 263 complete DC potentiostat/galvanostat, with Power suite software. A three electrode cell assembly containing mild steel and aluminum coupons of size 1x1 cm respectively, was embedded in a specimen holder as the working electrode (WE), the large area platinum mesh was serve as counter electrode (CE) and a saturated calomel electrode as reference electrode (RE). All electrochemical experiment was conducted at room temperature (25 0C) using 100 ml of electrolyte (1M HCl) with stationary condition. Before each potentiodynamic polarization (TAFEL) experiment, the electrode was allowed to corrode freely and it open circuit (OCP) was recorded as a function of time up to 15 minutes. After this time the steady state OCP, corresponding to corrosion potential (E corr) of the working electrode, was obtained. The potentiodynamic tafel measurement was starting from cathodic to the anodic direction, E = Ecorr ± 250mV with a scan rate of 1.0mVs’. These procedures were repeated for each concentration of JGPE (Loto et al., 2014) [9].
Fourier Transform Infrared (FTIR) analysis
FTIR analyses of the exudates gum was carried out using SHIMADZU (Model: FTIR-8400S) Fourier Transform Infrared Spectrophotometer. A portion of gum samples (2 g) was prepared by mixing thoroughly with 100 cm3 of anhydrous KBr to form disc before scanning through a wave number range of 4500-400cm-1 (Femi Oyewol et al., 2004) [10].
Scanning Electron Microscopy
The morphological features of the gums were studied with JSM-5600 LV scanning electron microscopy (SEM) from JEOL, Tokyo, Japan. The dried sample was mounted on a metal stub and sputtered with gold in order to make the sample conductive, and the image is taken at an accelerating voltage of 10KV (Femi Oyewol et al., 2004) [10].
RESULTS AND DISCUSSION
Weight loss study
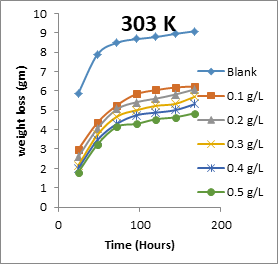
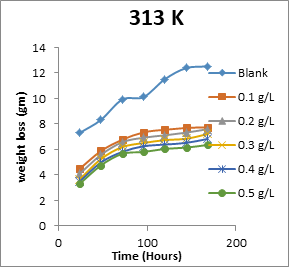
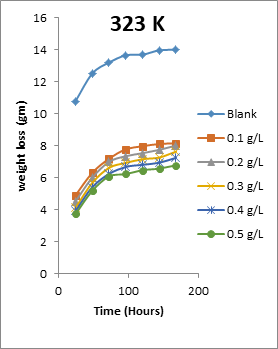
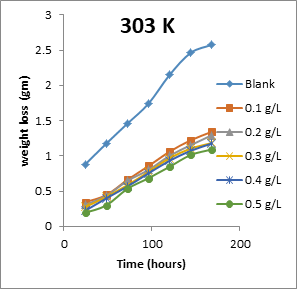
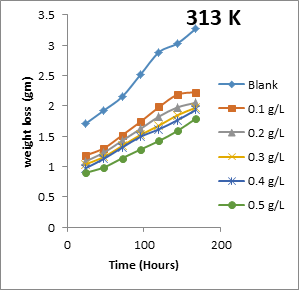
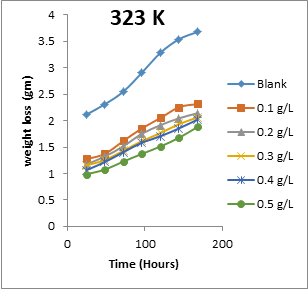
The results obtained for the variation of weight loss of metals with time during the inhibition of the corrosion of mild steel and aluminium in solutions of HCl by EA gum are presented in Figures 1 to 2.
Figure 1: Variation of weight loss with time for the corrosion of mild steel in 0.1 M HCl containing various concentrations of EA gum at 303, 313, 323 and 333 K.
Figure 2: Variation of weight loss with time for the corrosion of aluminium in 0.1 M HCl containing various concentrations of EA gum at 303, 313, 323 and 333 K.
Each of the figures also contains plots obtained at different temperatures (303 to 333 K). From the plots, it shows that the weight loss of both mild steel and aluminium increases with increase in the period of time and with increase in temperature indicating that the rate of corrosion of the metals increases with time and with increase in temperature. In all cases, weight loss of the metals decreases with increase in the concentration of the used EA gum indicating that the gum exudate is an adsorption inhibitor. For an adsorption inhibitor, weight loss is expected to increase with decrease in concentration (Ameh and Eddy, 2018) [11]. It is interesting to state that aluminium was found to show a lower weight loss than mild steel at the same experimental conditions, indicating that aluminium corroded less than mild steel in the various media. This may be due to aluminium forming an oxide on its surface which alongside with the adsorbed inhibitors made it less prone to attack by the acid corrodent (Ameh and Eddy, 2018) [12].
Corrosion rate of mild steel and aluminium in solutions of HCl at various concentrations of EA gum at 303 to 333 K are presented in Table 1.
Table 1: Rate of corrosion of mild steel and aluminium and Inhibition efficiencies of EA gum in 0.1 M HCl.
|
Concentration of Inhibitor |
Inhibition efficiency (%) |
Corrosion rate (mmpy) |
||
|
Mild Steel |
Aluminium |
Mild Steel |
Aluminium |
|
|
Blank at 303K |
|
- |
0.134 |
0.058 |
|
0.1 g/L |
49.2 |
53.4 |
0.068 |
0.027 |
|
0.2 g/L |
55.9 |
57.1 |
0.059 |
0.021 |
|
0.3 g/L |
63.4 |
62.8 |
0.049 |
0.017 |
|
0.4 g/L |
66.4 |
68.5 |
0.045 |
0.013 |
|
0.5 g/L |
69.4 |
80.0 |
0.041 |
0.008 |
|
|
|
|
|
|
|
Blank at 313K |
|
|
0.166 |
0.112 |
|
0.1 g/L |
38.5 |
30.3 |
0.102 |
0.078 |
|
0.2 g/L |
43.9 |
36.6 |
0.093 |
0.071 |
|
0.3 g/L |
50 |
39.2 |
0.083 |
0.068 |
|
0.4 g/L |
52.4 |
42.8 |
0.079 |
0.064 |
|
0.5 g/L |
54.8 |
47.3 |
3.303 |
0.898 |
|
|
|
|
|
|
|
Blank at 323K - |
|
|
0.246 |
0.139 |
|
0.1 g/L |
54.4 |
40.2 |
0.112 |
0.083 |
|
0.2 g/L |
58.1 |
44.6 |
0.103 |
0.077 |
|
0.3 g/L |
62.1 |
45.3 |
0.093 |
0.076 |
|
0.4 g/L |
63.8 |
49.6 |
0.089 |
0.070 |
|
0.5 g/L |
65.4 |
53.2 |
0.085 |
0.065 |
|
Blank at 333K |
|
|
0.251 |
0.154 |
|
0.1 g/L |
53.7 |
39.6 |
0.116 |
0.093 |
|
0.2 g/L |
57.3 |
43.5 |
0.107 |
0.087 |
|
0.3 g/L |
61.3 |
46.1 |
0.097 |
0.083 |
|
0.4 g/L |
62.9 |
48.7 |
0.093 |
0.079 |
|
0.5 g/L |
64.5 |
51.9 |
0.089 |
0.074 |
Electrochemical Study
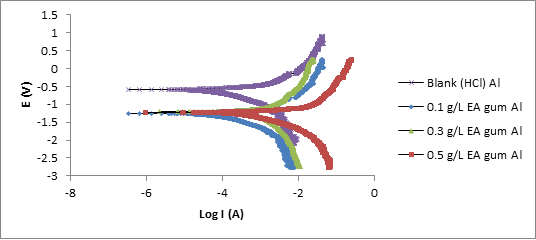
Potentiodynamic polarization (PDP) measurement was also carried out. Plots (Tafel plots) obtained for the variations of applied potential with log(current) for corrosion of the studied metals in the absence and presence of the EA gum are presented in Figures 3 to 4.
Figure 3: Potentiodynamic polarization curves for the mild steel in 0.1 M HCl in the absence and presence of different concentrations of EA gum exudate.
Figure 4: Potentiodynamic polarization curves for the Aluminium in 0.1 M HCl in the absence and presence of different concentrations of EA gum exudate.
Each of the plots consists of cathodic and anodic arms, as well as a plateau. The displacement shown by the cathodic and anodic arms of the plots seems to be extended for all the plots. The addition of the gum exudate is seen to affect both the cathodic and anodic partial reactions, thereby reducing the cathodic and anodic current densities and the corresponding corrosion densities. This indicates that EA gum acted as a are mixed type inhibitor (Ameh, 2018) [14].
Polarization parameters such as corrosion current density (icorr), anodic (βa) and cathodic (βc) slopes derived from the polarization curves by Tafel extrapolation at the corrosion potential (Ecorr) and are presented in Tables 2.
Table 2: Polarization data for the corrosion of the metal in 0.1 M HCl in the absence and presence of the studied inhibitor at 303 K.
|
Inhibitor |
Concentration (g/L) |
βa mVdec-1 |
βc mVdec-1 |
Ecorr (mV) |
Icorr (μA) |
I % |
|
Mild steel |
Blank |
117.2 |
120.6 |
- 603.2 |
140.25 |
- |
|
0.1 |
97.5 |
110.0 |
-544.3 |
34.52 |
75.39 |
|
|
0.3 |
95.2 |
106.2 |
- 542.0 |
22.82 |
83.73 |
|
|
0.5 |
94.1 |
104.7 |
-538.3 |
19.48 |
86.11 |
|
|
Aluminium |
Blank |
113.5 |
117.1 |
- 592.1 |
128.88 |
- |
|
0.1 |
90.5 |
92.3 |
- 565.2 |
43.18 |
66.50 |
|
|
0.3 |
84.4 |
90.3 |
- 555.5 |
28.28 |
78.06 |
|
|
0.5 |
82.1 |
88.3 |
- 550.2 |
26.56 |
79.39 |
These include geometric blocking through the adsorption of the inhibitor, active sites blocking by the adsorbed inhibitor and electro-catalytic effect of the inhibitor or its reaction products. From the polarization results, it can be inferred that the gum exudate acted via geometric blocking effect. The inhibition efficiencies of the inhibitor obtained from PDP measurement followed similar trends with one obtained from weight loss experiments. (Ameh, 2018) [14].
Effect of temperature
The effect of temperature on the corrosion of mild steel and aluminium in the studied media was investigated using the Arrhenius equation, (Eddy et al., 2018) [14],
From the logarithm of equation 4.1, equation 4.2 was obtained:
where CR is the corrosion rate of the metal (mild steel or aluminium, A is the Arrhenius or pre-exponential factor, R is the universal gas constant and T is the temperature. Equation 4 reveals that a plot of ln(CR) versus 1/T (plots not shown) is expected to be linear, with slope and intercept equal to Ea/R. Values of the activation energy calculated from the slope are presented in Table 4.
Table 3: Arrhenius parameters for the inhibition of the corrosion of the studied metals in 0.1 M HCl by EA gum.
|
Metal |
System |
Slope |
Intercept |
Ea (J/mol) |
A |
R2 |
|
Mild steel |
Blank |
1.5479 |
-3.7411 |
12.85221 |
0.021722 |
0.9956 |
|
0.1 g/L |
2.1055 |
-2.157 |
17.48197 |
0.000087 |
0.9902 |
|
|
0.2 g/L |
2.2315 |
-1.558 |
18.52814 |
0.000172 |
0.9804 |
|
|
0.3 g/L |
2.3947 |
-2.211 |
19.88319 |
0.000112 |
0.9868 |
|
|
0.4 g/L |
2.4101 |
-2.410 |
20.01106 |
0.000088 |
0.9984 |
|
|
0.5 g/L |
2.4826 |
-2.421 |
20.61303 |
0.000887 |
0.9991 |
|
|
Aluminium |
Blank |
1.4102 |
-4.313 |
11.70889 |
0.000716 |
0.9956 |
|
0.1 g/L |
1.6628 |
-3.319 |
13.80623 |
0.000348 |
0.9908 |
|
|
0.2 g/L |
1.9228 |
-2.245 |
15.96501 |
0.000885 |
0.9888 |
|
|
0.3 g/L |
2.1589 |
-2.112 |
17.92535 |
0.000588 |
0.9854 |
|
|
0.4 g/L |
2.3346 |
-1.724 |
19.38418 |
0.000768 |
0.9955 |
|
|
0.5 g/L |
2.4196 |
-1.239 |
20.08994 |
0.000984 |
0.9898 |
The activation energies ranged from 10 to 21 J/mol. These values are within the limit expected for the mechanism of physical adsorption. Therefore, the adsorption of EA gum on the surface of mild steel and aluminium support the mechanism of physical adsorption. It can also be deduced from Table 4, that the activation energy for the blank is lower than those calculated for the inhibited system, which indicated that the corrosion of mild steel / aluminium in solution of the acid is retarded by EA gum.
Adsorption isotherm
Adsorption isotherm is significant in analysing the adsorption behaviour of an inhibitor. The general form of adsorption isotherm equation is given as follows (Loto et al., 2014) [9].
where 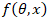
The adsorption data obtained from the weight loss study were used to test for the isotherm that best describes the adsorption of EA gum at various temperatures. The test revealed that the Temkin adsorption model fitted the adsorption of EA gum.
The Temkin adsorption isotherm is expressed by equation 7 which is obtained by rearranging and taking the logarithm of equation 6 (Eddy et al., 2014) [15].
The mathematical implication of equation 4.4 is that a plot of q versus logC should be linear with slope and intercept equal to 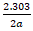

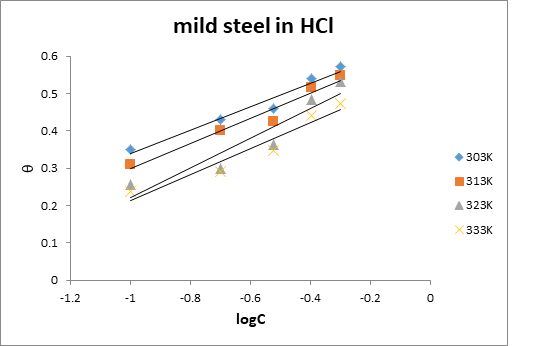
Figure 5: Temkin isotherm for the adsorption of EA gum on mild steel surface in 0.1 M HCl at various temperatures.
Figure 6: Temkin isotherm for the adsorption of EA gum on aluminium surface in 0.1 M HCl at various temperatures.
Shows Temkin isotherm for the adsorption of EA gum on the mild steel and aluminium surfaces in the studied media. Data obtained from the plots are presented in table 5 and 6.
Table 4: Temkin parameters for the inhibition of the corrosion of the studied metal in 0.1 M HCl by EA gum.
|
Metal |
System |
Slope |
Intercept |
a |
Log K |
R2 |
|
|
Mild steel |
303 K |
0.3215 |
0.6605 |
3.58165 |
2.054432 |
-10.615 |
0.9605 |
|
313 K |
0.3411 |
0.6475 |
3.37584 |
1.89827 |
-11.116 |
0.9552 |
|
|
323 K |
0.4112 |
0.6327 |
2.80030 |
1.538667 |
-11.474 |
0.8999 |
|
|
333 K |
0.4217 |
0.5817 |
2.73061 |
1.379417 |
-11.688 |
0.9492 |
|
|
Aluminium |
303 K |
0.3335 |
0.6754 |
3.45277 |
2.025187 |
-10.751 |
0.9894 |
|
313 K |
0.3256 |
0.6043 |
3.53655 |
1.855958 |
-10.956 |
0.9651 |
|
|
323 K |
0.2758 |
0.5448 |
4.17513 |
1.975344 |
-11.174 |
0.9562 |
|
|
333 K |
0.2562 |
0.5106 |
4.49454 |
1.992974 |
-11.465 |
0.9415 |
The data reveal that high degrees of linearity were observed and that interaction parameters were positive. Generally positive values of ‘a’ indicates attraction between the adsorbed species while negative values points toward repulsion (Ameh, 2012) [16]. Therefore, attraction is involved in the adsorption layer and that there is an increase in the surface energy even as the degree of surface coverage increases.
It is significant to note that the equilibrium constant of adsorption obtained from the Temkin adsorption model (Kads) is related to the standard free energy of adsorption according to equation 8 (Eddy, 2011) [6]
All parameters in equation 8 are as described earlier. Values of 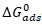
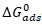
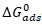
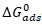
FTIR STUDY
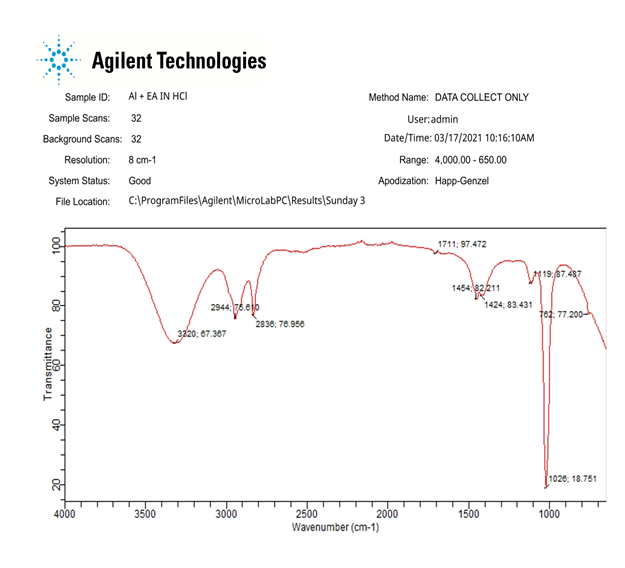
The FTIR spectra of metal, EA gum powder as well as scrapings from the inhibitor films on the surface of mild steel and aluminum specimens immersed for 7 days in the corrodent are presented in Figures 7 to 8. Table 7 and 8 present the various peaks intensities and the corresponding functional groups responsible.
(B)
Figure 7: FTIR spectrum of the corrosion product of (A) mild steel and (B) Aluminium dipped in 0.1 M HCl and in the absence of inhibitor.
Figure 8: FTIR spectrum of the corrosion product of Aluminium dipped in 0.1 M HCl and in the presence of the inhibitor.
Table 5: Frequencies and peak intensities of FTIR absorption by EA and the corrosion product of mild steel in the presence of EA.
|
EA gum before adsorption |
Corrosion product of mild steel in the presence of EA after adsorption in HCl medium |
Functional group/assignment |
||
|
Frequency (cm-1) |
Intensity |
Frequency (cm-1) |
Intensity |
|
|
|
|
|
|
C-H rocking |
|
821 |
57.566 |
|
|
=C-H “oops” bending stretching |
|
925 |
68.116 |
|
|
Carbon ring vibration |
|
1037 |
39.483 |
1045 |
81.860 |
C-O stretch |
|
|
|
1182 |
80.896 |
C – N stretch |
|
1365 |
68.297 |
1346 |
86.038 |
NO2 symmetric stretch |
|
1413 |
70.007 |
1495 |
87.330 |
Aromatic C=C stretch |
|
1514 |
81.105 |
|
|
Aromatic C-H stretch |
|
1640 |
66.038 |
|
|
C=O symmetric stretch |
|
|
|
|
|
C=O asymmetric stretch |
|
2121 |
97.449 |
2124 |
96.097 |
Carboxylic acid OH stretch |
|
|
|
|
|
C-H stretch |
|
2936 |
75.848 |
2908 |
20.460 |
C-H stretch |
|
3257 |
46.616 |
3268 |
45.102 |
overtone from C=O peak |
Table 6: Frequencies and peak intensities of FTIR absorption by EA and the corrosion product of aluminium in the presence of EA.
|
EA gum before adsorption |
Corrosion product of aluminium in the presence of EA after adsorption in HCl medium |
Functional group/assignment |
||
|
Frequency (cm-1) |
Intensity |
Frequency (cm-1) |
Intensity |
|
|
|
|
|
|
C-H rocking |
|
|
|
|
|
C-H rocking |
|
821 |
57.566 |
|
|
=C-H “oops” bending stretching |
|
925 |
68.116 |
|
|
Carbon ring vibration |
|
|
|
|
|
C-O vibration |
|
1037 |
39.483 |
1026 |
18.751 |
C-O stretch |
|
|
|
1119 |
87.487 |
|
|
1365 |
68.297 |
|
|
NO2 symmetric stretch |
|
1413 |
70.007 |
1424 |
83.431 |
Aromatic C=C stretch |
|
1514 |
81.105 |
|
|
Aromatic C-H stretch |
|
1640 |
66.038 |
1711 |
82.211 |
C=O symmetric stretch |
|
|
|
|
|
C=O asymmetric stretch |
|
2121 |
97.449 |
2120 |
20.260 |
Carboxylic acid OH stretch |
|
|
|
|
|
C-H stretch |
|
2936 |
75.848 |
2836 |
76.956 |
C-H stretch |
|
3257 |
46.616 |
3320 |
67.367 |
overtone from C=O peak |
From the spectra of the corrosion products obtained for the corrosion of mild steel and Aluminum in HCl solutions (Figure 7a and 7b), it can be seen that the corrosion product without the inhibitor indicate only the functional groups present in the metal which shows that EA gum was not present, therefore there was no adsorption.
Comparison of the FTIR spectrum of the EA gum (Figures 8) to the corrosion product of metal when EA gum was used as an inhibitor in HCl and NaOH, it can be observed that most of the peaks for the EA gum were also noticed in the surface films of the inhibitors on the studied metal surfaces, suggesting that most of the functional groups within the gum exudate are also present in the surface film. However, a shift in the wave number of absorption of the gum for C-O stretch, NO2 symmetric stretch and aromatic C=C stretch were observed in the corrosion product of the metal when EA gum was used as an inhibitor indicating an interaction between the inhibitor and the surface of mild steel and aluminum. There was disappearance of some absorption bands of the inhibitor like “the carbon ring vibration and the aromatic C-H stretch” after inhibition. Also, new IR absorption bands were formed including C - N stretch. The absence of some IR absorption bands and the formation of new one clearly reveal that the functional groups or bonds were used for the adsorption of EA on the surface of metal.
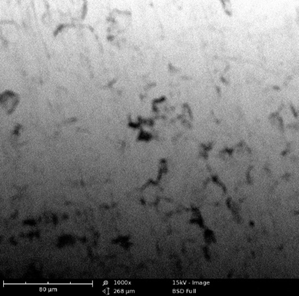
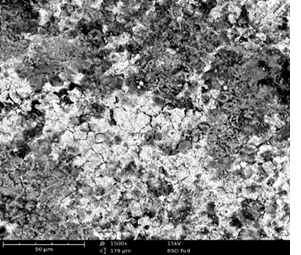
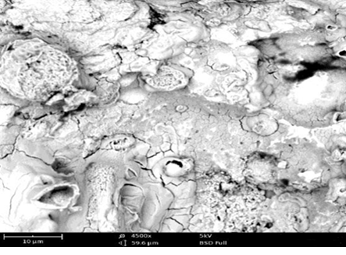
Scanning Electron Microscopy (SEM) Study
Surface morphologies of mild steel and aluminum in 0.1 M solutions of HCl were studied using scanning electron microscopy.
Plate 1: Pure Aluminum Plate 2: Pure Mild steel
Plate 3: MildSteel in 0.1M HCl) Plate 4: Aluminum in 0.1 M HCl
(Absence of Inhibitor) (Absence of Inhibitor)
Plate 5: Aluminium in EA gum + 0.1M HCL Plate 6: Mildsteel in EA gum + 0.1M HCL
(Presence of Inhibitor) (Presence of Inhibitor)
Plates 1 – 6 show the micrographs of the corroded mild steel and aluminum in the presence and absence of the EA gum, it could be observed that the morphologies obtained for the polished mild steel and aluminum were smooth. However, the morphologies obtained for those immersed in the acidic media without inhibitor (Plates 3 – 4) were badly attack with evident of pits and cracks by the corrosive environment when compared to those immersed in the media containing inhibitors (Plates 5–6). This means that good protective adsorbed films were formed on the metal coupons and the smother surfaces with little pits and cracks observed in the presence of the EA gum implies the corrosion rate was lowered by the inhibitor. The pattern of the formation of the thin protective covering as revealed by the SEM results is consistent with the inhibition efficiencies values obtained from the potentiodynamic polarization/ weight loss measurements (Ameh, 2012) [16].
CONCLUSION
This work encompasses biochemical extraction and characterization of two plant extracts, formulation of gum exudates from Erythrophelum africanum into inhibitor and testing the formulated inhibitor for the protection of mild steel and aluminum HCl media. Based on the results obtained the following conclusions could be drawn:
- Erythrophelum africanum gum is a corrosion inhibitor for mild steel and aluminum in solution of HCl
- The weight loss experiments showed decreased corrosion rates with increase in inhibitor concentration and exposure time. Potentiodynamic studies showed higher value of inhibition efficiencies percentages
- The inhibition efficiencies were observed to increase with increase in concentration of inhibitors for both gravimetric based mass loss experiments and electrochemical measurements.
- Potentiodynamic polarization curves reveal that the inhibitor affected both anodic and cathodic processes, i.e., it is a mixed-type inhibitor.
- The adsorption of the inhibitor onto the studied metal surfaces were exothermic and spontaneous, favoured the mechanism of physical adsorption and is best described by the temkim adsorption model.
REFERENCES
- Ameh PO. (2021). Study of the Functional Groups Associated with the Corrosion Inhibition of Stainless Steel Arch Bar in Acidic Medium by Khaya Grandifolia Gum Exudate. Communicat Phy Sci. 7(4): 384-391.
- Hosseini SMA, Eftekhar S, Amiri M. (2007). Polarization behavior of stainless steel type 302 in HCL solution of benzotriazole. Asian J Chem. 19(4): 2574-2580.
- Eddy NO, Ebenso EE. (2008). Adsorption and inhibitive properties of ethanol extract of Musa sapientum peels as a green corrosion inhibitor for mild steel in acidic medium. Afri J Pure Appl Chem. 2(6):046-054.
- Eddy NO, Odoenmelam SA, Odiongengi AO. (2009b) Ethanol extract of Musa species peel as a green Corrosion inhibitor for mildsteel: Kinetics, adsorption and thermodynamic consideration. Electronic J Envir Agri Food Chem:246-257.
- Ameh PO, Odiongenyi AO, Eddy NO. (2012). Joint effect of Anogessiusleocarpus gum (AL gum) exudate and halide ions on the corrosion of mild steel in 0.1 M HCL. Portugaliae Electrochimica Acta. 30(4):235-245.
- Eddy NO. (2011). Experimental and theoretical studies on some amino acids and their potential activity as inhibitors for the orrosion of mild steel, part 2. J Adv Res. 2:35-47
- Ameh PO. (2012). Physciochemical and Rheological studies on some natural polymers and their potential as corrosion inhibit0r. London: lambert Academic publishing:26-32.
- Eddy NO. (2010). Adsorption and inhibitive properties of ethanol extract of Garcinia Kola and Colonitida for the corrosion of mild steel in H2S04. Pigment Resin Technol. 39(6):347-353.
- Loto RT, Loto CA, Popoola APL. (2014). Electrochemical Studies of the Corrosion Inhibition Effect of 2 – Amino – 5 – Ethyl – 1, 3, 4 – Thiadiazole in on Low Carbon Steel in Dilute Sulphuric acid. J Chem Soc Pak. 36(6):1043-1051.
- Femi-Oyewol M, Musilui O. Taiwao O. (2004). Evaluation of the suspending properties of Albiziazygia gum on sulphadimidine suspension. Tropical Pharm Res. 3(1):279-284.
- Eddy NO, Ameh PO, Essien NB. (2018). Experimental and computational chemistry studies on the inhibition of aluminum and mild steel in 0.1 M HCl by 3 nitrobenzoic acid. Journal of Taibah Uni Sci. 12(5):545-556
- Ameh PO, Eddy NO. (2018). Theoretical and Experimental Investigations of the Corrosion Inhibition Action of Pilio Stigma Thonningii Extract on Mild Steel in Acidic Medium. J Communicat Phy Sci. 3(1):27–42.
- Eddy NO, Abechi SE, Ameh PO, Ebenso EE. (2013b). GCMS, FTIR, SEM, Physiochemical and Rheological Studies on Albizia Zygia Gum. Walailak J Sci Tech. 10 (3):247–265.
- Ameh PO. (2018). Electrochemical and computational study of gum exudates from Canarium schweinfurthii as green corrosion inhibitor for mild steel in HCl solution. J Taibah Univ Sci.12(6):783-795.
- Eddy NO, Momoh-Yahaya H, Oguzie EE. (2014). Theoretical and experimental studies on the corrosion inhibition potentials of some purines for aluminum in 0.1 M HCl. J Adv Res. 6(2):203-217.
- Ameh PO. (2012). Physciochemical and Rheological studies on some natural polymers and their potential as corrosion inhibit0r. London: lambert Academic publishing:26-32.
 Abstract
Abstract  PDF
PDF
.PNG)