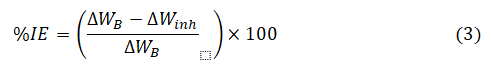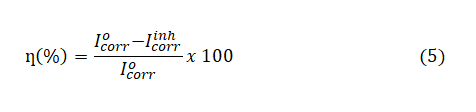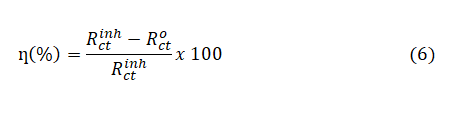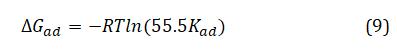Current Issue
Adsorption and corrosion inhibition behavior of Acanthus montanus leaves Extract on Mild Steel in Sulphuric acid Medium
Cookey GA1, Udom I G1,*, BoEkom EJ2, Paul O Ameh3
1Physical Chemistry Unit, Department of Chemistry, Rivers State University, Port Harcourt, Rivers States, Nigeria
2Physical Chemistry Unit, Department of Chemistry, University of Uyo, Akwa Ibom state, Nigeria
3Physical Chemistry Unit, Department of Chemistry, Nigeria Police Academy Wudil, Kano, Nigeria
*Corresponding Author: Udom I G, Physical Chemistry Unit, Department of Chemistry, Rivers State University, Port Harcourt, Rivers States, Nigeria; Email: [email protected]
Received Date: September 12, 2023
Publication Date: May 07, 2024
Citation: Cookey GA, et al. (2024). Adsorption and corrosion inhibition behavior of Acanthus montanus leaves Extract on Mild Steel in Sulphuric acid Medium. Catalysis Research. 3(4):16.
Copyright: Cookey GA, et al. © (2024).
ABSTRACT
The inhibitive action and mechanism of Acanthus montanus leaves extract (AMLE) for the corrosion of mild steel in 2M H2SO4 was investigated using gravimetric, Potentiodynamic polarization (PDP), electrochemical impedance spectroscopy (EIS), Fourier transform infrared (FTIR), and Scanning electron microscope (SEM) techniques. It was found that the extract inhibits the acid induced corrosion of mild steel. The inhibition efficiency (%IE) was observed to increase with increasing extract concentration but decreased with rise in temperature. Maximum inhibition efficiency value of 75.7% was obtained at 0.5g/l from gravimetric measurement. Electrochemical measurements results showed that AMLE acted as a mixed-type inhibitor. The adsorption of AMLE on mild steel was physical and obeyed Langmuir adsorption isotherm model. Kinetic data followed a first-order reaction type. Results obtained from the gravimetric, potentiodynamic polarization and impedance measurements showed similar trends of inhibition efficiency. Surface analysis by FTIR and SEM confirmed the formation of protective layer on the mild steel surface.
Keywords: Mild steel, Corrosion inhibition, Acanthus montanus, sulphuric acid
INTRODUCTION
Iron and its alloys particularly mild steel are extensively used in various industries because of its low cost and material properties that are acceptable in many applications such as chemical, petroleum, food and engineering industries [1]. However, its usefulness is jeopardized by corrosion when exposed to aggressive environments such as acids which are generally used in industry for cleaning, pickling and de-scaling of metals [2]. Hence, protection of metals from corrosion is very important especially in industries where acid media are used for one purpose or the other. It has been reported [3,4] that the best method to mitigate the corrosion of metals in acid media is the introduction of corrosion inhibitor into the corrosive medium. Previously, synthetic inhibitors and inorganic related inhibitors such as compounds containing borates, chromate, dichromate, nitrite, nitrate, phosphates and other heavy metals were used as corrosion inhibitors for metals in acid and alkaline media [5,6]. Due to severe environmental regulations and economic benefits, a number of plant extracts and other natural products that are biodegradable, non-toxic and low cost are used as alternative corrosion inhibitors [3,7,8]. A number of plant extracts such as red peanut skin extract and furfural [9], Nicotiana tobacum leaves [10], Ephedra alata leaves [11], Dodonaeaviscosa leaves [12], Hibiscus sabdariffa leaves [13], Nypa frutican swurmbs leaves [14], Hyptis suaveolens leaves [15], stem, bark of Ficus asperifolia [16], Hunteria umbellata seed husk [17], Delonix regia leaves [18] and other natural occurring products such as Honey [19] have been reported as effective corrosion inhibitors for mild steel in different media. The successful results obtained from previous studies on naturally occurring substances as corrosion inhibitors for metals in acidic and alkaline environments have motivated this investigation on the efficiency of methanol extract of Acanthus montanus leaves as corrosion inhibitor for mild steel in 2M H2SO4 solution.
Acanthus montanus commonly known as leopard tongue or mountain thistle is a perennial plant with an average height of 1.5meters. The leaves are dark glossy green, deeply lobed and toothed with short spines. Acanthus montanus plant is wide spread in Africa and has several medicinal values. It is used in South-Western Nigeria for treatment of various ailments, like stomach upset, urinary disorders, etc. Fresh leaves extract has been reported in folk medicine to relieve aches, pains, cough, epilepsy, inflammation, intestinal helminthaisis, dysmenorrhea, false labour and threatened abortion [20-22]. The plant parts are biodegradable and renewable. Phytochemical screening conducted on methanolic extract of Acanthus montanus leaves by [23] reveal that it contains alkaloids, saponins, tannins, flavonoids, glycosides, carbohydrates, terpenoids and fixed oil. Hence, Acanthus montanus leaves are rich in several biodegradable, eco-friendly heterocyclic compounds. This research investigates the efficacy of methanol extract of Acanthus montanus leaves in corrosion inhibition of mild steel in 2.0M H2SO4 solutions using weight loss, potentiodynamic polarization, electrochemical impedance, FT-IR and SEM techniques. Thermodynamic and kinetic data are also evaluated.
MATERIALS AND METHODS
Materials Preparation
Corrosion tests were performed on mild steel sheets with weight percentage (Wt %) composition as follows: C=0.17, Mn=0.46, P-0.047, S=0.017, Cu=0.05, Si=0.26 and Fe=99.35. The mild steel sheets were mechanically press cut into 4x2 cm for weight loss measurements and 2 x1.5 cm for electrochemical measurements. The coupons were polished with emery paper of variable grades starting with the coarsest and then proceeding in steps to the finest grade, washed in distilled water, degreased in ethanol, dried in acetone and then stored in a moisture free desiccator prior to analysis. The corrosive medium was 2M H2SO4 prepared from 98% analytical grade supplied by Sigma–Aldrich and doubly distilled water was used for the preparation of all reagents.
Preparation of Plant Extract
The fresh leaves of Acanthus montanus plant were collected from a farm land in Oruk-anam, Akwa Ibom State, South-South Nigeria. The leaves were washed thoroughly with deionized water to remove impurities, sun-dried to constant weight and ground to powder using an electric blender. 50 grams of the powdered sample was extracted using 1 litre of 99.8% methanol in the soxhlet extractor. The resulting extract was evaporated to near dryness in an oven at 40°C to obtain a paste residue devoid of methanol. The extract was then weighed, stored in sample containers for use. Different concentrations (0.1 - 0.5) grams of the extract were dissolved in 250 ml of 2M H2SO4 solution and used for the corrosion studies.
Weight Loss Method
Weight loss measurements were conducted under total immersion of already polished and weighed mild steel coupons in 100 ml of each test solution of a giving concentration of Acanthus montanus leaves extract and in blank solution at temperatures ranged 303–353K. A thermostated water bath was used to regulate the temperatures. The coupons were retrieved from the test solutions after every 2 hrs progressively for 18 hrs, washed in double distilled water, dried and then reweighed. All tests were carried out in triplicate in order to get good reproducibility.
The weight loss was calculated in grams as the difference between the initial weight prior to immersion and weight after removal of the corrosion product. The weight loss measurement is in accordance with [24] as stated in Equation 1.
where, ΔW(g) is the weight loss of the mild steel coupons, Wi is weight before immersion, while Wf is the weight after immersion in the test solution.
From the weight loss (∆W) results, the corrosion rate (CR), percentage inhibition efficiency (%IE) and degree of surface of coverage (θ) were calculated using equation 2, 3 and 4 respectively.
where, CR is corrosion rate, ∆W is weight loss (g), A is the area of the coupon in cm2 and t is time of immersion (hrs).
The inhibition efficiency (%IE) was computed using the equation:
The degree of surface coverage (θ) was computed from,
where, ∆WB and ∆Winh are the weight loss of the mild steel in the uninhibited and inhibited solutions respectively.
Electrochemical Methods
Electrochemical studies were carried out in the conventional three-electrode Pyrex cell with a platinum as counter electrode (CE), the reference electrode was a saturated calomel electrode (SCE), which was coupled to a Luggin capillary. Mild steel coupons with 0.5cm2 exposed areas were used as the working electrode (WE). The experiments were performed using a VERSASTAT 4 Princeton Applied electrochemical analyzer. All electrochemical measurements were carried out at 303K using 100ml test solution in stationary condition. Before each potentiodynamic polarization (PDP) and electrochemical impedance spectroscopy (EIS) measurement, the working electrode was immersed in test solution for 30mins until a steady state open circuit potential (Eocp) was established. After measuring the open circuit potential, potentiodynamic polarization curves were then obtained with a scan rate of 1 mV s−1 in the potential range from -0.25 to +0.25 mV relative to the Eocp. Corrosion current density (Icorr) values were obtained by extrapolation of anodic and cathodic (βc and βa) Tafel lines to corrosion potential. The corrosion inhibition efficiency was evaluated from Icorr values by using the relationship;
where, η(%) is the percentage inhibition efficiency, 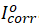
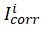
The electrochemical impedance spectroscopy (EIS) measurements were performed in a frequency range of 100kHz to 10mHz using amplitude of 5mV peak to peak with ac signals at open circuit potential. The value of Rct was calculated from Nyquist plot and the inhibition efficiency (ƞ%) was computed from the charge transfer resistance Rct values using the relationship;
where,  and
and  are the uninhibited and inhibited charge transfer resistance respectively. The double layer capacitance (Cdl) values were calculated using equation;
are the uninhibited and inhibited charge transfer resistance respectively. The double layer capacitance (Cdl) values were calculated using equation;
where, fmax is the frequency at which the imaginary component of the Nyquist plot is maximum. Each experiment was performed in triplicate to ensure reproducibility.
Surface Investigations
Fourier trans-form infra-red (FT-IR) spectroscopy
FTIR analysis of Acanthus montanus leaves extract and that of the corrosion products in the presence of the extracts was carried out using SHIMADZU 8400s, Japan spectrophotometer. The corrosion product of the analysis was obtained by immersing the mild steel coupons in 100ml of 2M H2SO4 solution containing optimum concentration (0.5g/l) of the extract. The coupons were left in the test solution for 18hrs, then removed and the film formed over the surface carefully collected, mixed thoroughly with KBr, made into pellets and then analyzed using FTIR.
Scanning Electron Microscopy (SEM) studies
The morphology of the mild steel coupons was examined before and after 18 hours exposure to 2M H2SO4 solutions in the absence and presence of the extract. Scanning electron microscope (SEM) model JSM-5600 LV was used for this investigation.
RESULTS AND DISCUSSION
Weight Loss Measurements
The Effect of extracts concentration and temperature on corrosion rate
The various corrosion parameters evaluated from weight loss measurements for mild steel in 2M H2SO4 solution without and with different concentrations AMLE are given in Table 1.
Results show that values of corrosion rate are lower in the presence of the extract compared with the blank. A marked decrease in corrosion rate was observed as concentration of the extract increased. The decrease in corrosion rate on increasing the extract concentration seems to point out simple adsorption behavior [25]. The corrosion rate also increased with increase in temperature signifying desorption of adsorbed protective layer at higher temperature. According to [17], at elevated temperature, reacting molecules collide faster leading to more consumption of the reactant and formation of the product.
Table 1: Calculated values of corrosion rate (CR), inhibition efficiency (IE) and surface.
|
CR |
%IE |
Surface coverage |
|||||||||||||
|
Conc. |
(gcm-2hrs-1)x 10-4 |
(θ) |
|||||||||||||
|
(g/l) |
|||||||||||||||
|
303K |
313K |
323K |
333K |
353K |
303K |
313K |
323K |
333K |
353K |
303K |
313K |
323K |
333K |
353K |
|
|
Blank |
13.16 |
17.39 |
18.62 |
20.08 |
23.33 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
0.1 |
5.17 |
7.18 |
8.07 |
9.71 |
11.96 |
60.73 |
58.74 |
56.67 |
51.67 |
48.71 |
0.607 |
0.587 |
0.566 |
0.516 |
0.487 |
|
0.2 |
4.53 |
6.7 |
7.51 |
8.94 |
11.01 |
65.67 |
61.51 |
59.7 |
55.65 |
52.82 |
0.656 |
0.615 |
0.597 |
0.556 |
0.528 |
|
0.3 |
4 |
6.13 |
7.12 |
8.01 |
10.08 |
69.63 |
64.74 |
61.76 |
59.76 |
56.82 |
0.696 |
0.647 |
0.617 |
0.597 |
0.568 |
|
0.4 |
3.61 |
5.62 |
6.38 |
7.28 |
9.18 |
72.6 |
67.74 |
65.73 |
63.75 |
60.82 |
0.726 |
0.677 |
0.657 |
0.637 |
0.608 |
|
0.5 |
3.19 |
4.92 |
5.84 |
6.68 |
8.44 |
75.78 |
71.74 |
68.68 |
66.76 |
63.84 |
0.757 |
0.717 |
0.686 |
0.667 |
0.638 |
coverage (θ) for mild steel in 2M H2SO4 solution with and without various concentrations of ACML extract at 303K to 353K.
Effect of extract concentration and temperature on inhibition efficiency
The results presented in Figure 1 and Table 1 show the inhibition efficiency of various concentrations of the extract on mild steel in H2SO4 acid solution. The inhibition efficiency is observed to increase with increase in concentration of the extract. This is a reflection of the increase in surface coverage with concentration of the extract. Thus, a good correlation exists between %IE and the adsorbent surface covered by the adsorbed extract molecules. Conversely, inhibition efficiency and surface coverage values reduced with increase in temperature. Gradual desorption of the adsorbed extract molecules on the mild steel surface is suspected which would have led to the exposure of the adsorbent surface to the corrosive environment at higher temperatures. According to [26]; decrease in inhibition efficiency with increase in temperature indicates physical adsorption.
Figure 1: Variation of inhibition efficiency (%IE) with extract concentration (g/l) at different temperatures (K).
Adsorption Considerations
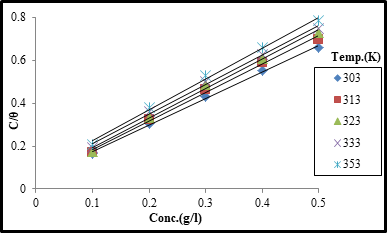
Adsorption isotherms provide basic information on the interaction between inhibitor molecules and metal surface [15]. The surface coverage (θ) data obtained from weight loss measurement was used to evaluate the best isotherm. Correlation coefficient values (R2) obtained from the plots of different adsorption isotherms such as Freundlich, Langmuir and Temkin were used to determine the best fit isotherm. The best fit was obtained with Langmuir isotherm which is expressed in equation 8.
where C is the concentration of the inhibitor, Kad is the equilibrium constant of adsorption- desorption process and θ is degree of surface coverage of the inhibitor on the mild steel.
Figure 2: Langmuir adsorption plot (C/θ vs C) of AMLE in 2M H2SO4 on mild steel at 303-333K.
Straight lines were obtained from the plot of C/θ vs C as shown in Figure 2. This suggests that adsorption of the extract molecules on the mild steel surface conformed to Langmuir isotherm. Values of adsorption parameters deduced from the plots are presented in Table 2. The results showed that the slopes and R2 values are approximately unity indicating strong adherence to the Langmuir adsorption isotherm. From the intercepts of the straight lines, the values of the Kad were computed. The free energy of adsorption (∆Gad) was calculated using equation 9.
where, R is the gas constant, T is the temperature, and 55.5 is the molar concentration of water in solution.
The values of Kad and ∆Gad are presented in Table 2. Table 2 shows decrease in the values of Kad as temperature increases. Similar observation has been reported [10,27]. This indicates that adsorption of AMLE on the mild steel surface was unfavorable at higher temperatures. The negative values of ∆Gad indicate that the reaction was spontaneous and AMLE was adsorbed on the mild steel surface through physical adsorption mechanism [28]. It has been stated [29, 30] that values of ∆Gad lower than or approximately -20kJmol-1 are consistent with electrostatic interaction between charged molecules and a charged metal surface and results in physical adsorption. Those higher than or approximately equal to -40kJmol-1 involve charge sharing or transfer from organic molecules to the metal surface to form a coordinate type of bond indicating chemical adsorption process.
Table 2: Langmuir isotherm parameters for the adsorption of AMLE on mild steel surface in H2SO4 solution at 303 - 353K.
|
T(K) |
R² |
Slope |
Kad |
-ΔG (kJ/mol)
|
|
303 |
0.997 |
1.236 |
1.292 |
10.766 |
|
313 |
0.995 |
1.319 |
1.276 |
11.087 |
|
323 |
0.995 |
1.376 |
1.276 |
11.442 |
|
333 |
0.995 |
1.381 |
1.143 |
11.491 |
|
353 |
0.995 |
1.435 |
1.097 |
12.061 |
Thermodynamics study
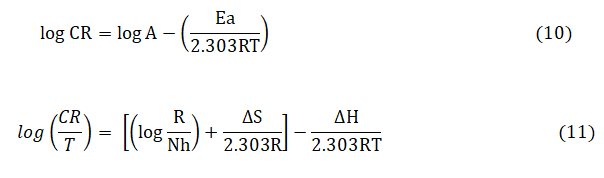
The Arrhenius-type equation (10) and Transition state equation (11) were used to calculate the activation parameters such as Activation Energy (Ea), Enthalpy (ΔH) and Entropy of activation (ΔS) for the corrosion reaction of mild steel in the absence and presence of different concentrations of AMLE.
where, R is the universal gas constant, A the Arrhenius pre-exponential constant, h is the plank’s constant and N, the Avogadro’s number.
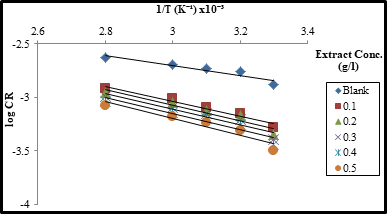
A plot of log CR vs (Fig. 3) gave straight lines with slope
(Fig. 3) gave straight lines with slope  from where the values of Ea were computed. The plot of log
from where the values of Ea were computed. The plot of log vs
vs  Fig not shown generated a linear graph with slope
Fig not shown generated a linear graph with slope  and intercept of
and intercept of  from where the values of ∆H and ∆S were calculated respectively.
from where the values of ∆H and ∆S were calculated respectively.
Figure 3: Arrhenius plot of log CR vs at different concentrations of AMLE for the corrosion of mild steel coupons in H2SO4 solution.
at different concentrations of AMLE for the corrosion of mild steel coupons in H2SO4 solution.
The calculated values of apparent activation energy (Ea), activation enthalpies (∆H) and entropies (∆S) are reported in Table 3. Values of Ea are positive and increased as concentration of the extract increased. The higher values of Ea in the presence of the extract indicate that the corrosion of mild steel in H2SO4 solution was inhibited by AMLE through physical adsorption process [30. 31]. The positive values of ∆H in both the absence and presence of the extract reflects the endothermic nature of the mild steel dissolution process. The increasing values with extract concentration signify that dissolution of the mild steel was slower in the presence of the extract [29, 32]. The negative values entropy of activation (∆S) in the presence and absence of the extract imply that the activated complex in the rate determining steps represent association rather than dissociation step. This suggests a decline in disorderliness from reactant to activated complex [1, 8].
Table 3: Activation parameters for the corrosion of mild steel in the absence and presence of AMLE in 2M H2SO4 solution.
|
Extracts Conc. (g/l) |
Ea (kJ/mol) |
∆Ha (kJ/mol) |
─∆Sa (J/mol) |
|
Blank |
8.62 |
6.09 |
145.30 |
|
0.1 |
13.36 |
10.74 |
137.68 |
|
0.2 |
13.82 |
11.28 |
136.76 |
|
0.3 |
14.15 |
11.62 |
136.48 |
|
0.4 |
14.21 |
11.68 |
136.55 |
|
0.5 |
15.01 |
12.46 |
135.56 |
Kinetic Study
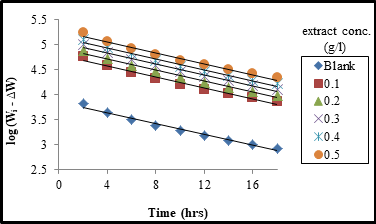
Figure 4 shows the plot of log (Wi – ∆W) versus time (hrs) for mild steel corrosion in 2M H2SO4 solution containing different concentrations of AMLE. Linear variations with correlation coefficient (R2) value close to unity were obtained at all temperatures studied. This indicates a first order reaction kinetics with respect to the corrosion of mild steel in the absence and presence of the extract. Similar observations have been reported [33-35].
The values of the rate constant (k) at the different extract concentrations were obtained from the slopes of the plots in Figure 4. From the values of rate constant, the half-life (t½ ) values of the metal in the test solutions were calculated using Equation 12;
The computed values are presented in Table 4. From the table, values of rate constant increased with temperature but decreased as the concentration of the AMLE increased. On the other hand, the half-life values decreased with rise in temperature and increased with increase extract concentration. This implies that the rate of corrosion increased with temperature probably due to enhanced kinetic energy of both adsorbent and adsorbate molecules but decreased with extract concentration due to its inhibiting property [23].
Figure 4: The variation of log (Wi - ∆W) with time (hrs) for the corrosion mild steel coupons in 2M H2SO4 solution with and without the leaves extract at 303K.
Table 4: Calculated values of rate constant (k) and half−life for mild steel in 2M H2SO4 solution containing AMLE at different temperatures (K)
|
Extract Conc.(g/l) |
Rate Constant, k ( hrs-1 ) x 10-3 |
Half-life, t½ (hrs) |
||||||||
|
|
303 |
313 |
323 |
333 |
353 |
303 |
313 |
323 |
333 |
353 |
|
Blank |
2.857 |
2.917 |
3.046 |
3.153 |
3.825 |
0.243 |
0.238 |
0.228 |
0.220 |
0.181 |
|
0.1 |
0.969 |
1.289 |
1.322 |
1.495 |
1.975 |
0.715 |
0.538 |
0.525 |
0.465 |
0.351 |
|
0.2 |
0.844 |
1.186 |
1.225 |
1.371 |
1.819 |
0.821 |
0.584 |
0.566 |
0.505 |
0.381 |
|
0.3 |
0.744 |
1.052 |
1.168 |
1.235 |
1.678 |
0.931 |
0.659 |
0.593 |
0.561 |
0.413 |
|
0.4 |
0.673 |
0.956 |
1.043 |
1.112 |
1.516 |
1.030 |
0.725 |
0.664 |
0.623 |
0.457 |
|
0.5 |
0.601 |
0.832 |
0.958 |
1.009 |
1.364 |
1.153 |
0.833 |
0.724 |
0.686 |
0.508 |
Electrochemical Study
Potentiodynamic polarization measurements
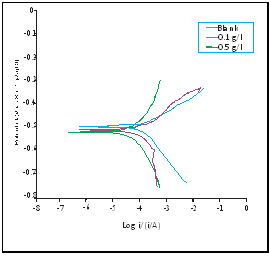
Figure 5 shows the potentiodynamic polarization curves obtained for mild steel in 2M H2SO4 solution in the absence and presence of different of concentrations AMLE at 303K. Tafel extrapolations of the anodic and cathodic lines of the polarization curves were used to calculate polarization parameters such as anodic and cathodic Tafel slopes (βc and βa), corrosion potential (Ecorr), and corrosion current density (Icorr) as shown in Table 5. The inhibition efficiency ( %) was calculated using Eq.5. From the results, no significant shift was noted on Ecorr values. If the shift in (Ecorr) values for the inhibited solution is more than 85mV with reference to the Ecorr of the blank, then the inhibitor could be classified as anodic or cathodic type and if the shift is below 85mV, the inhibitor might be regarded as a mixed type inhibitor [36, 37]. This indicates that AMLE acts as mixed-type inhibitor. The results also showed that Icorr values decreased with increase in concentration of the extract. This indicates that the extract molecules were adsorbed on the mild steel surface and thus inhibits the corrosion of the metal in H2SO4 solution. Further inspection of the results revealed that values of βa and βc change slightly in the presence of the extract, signifying that the extract control both anodic and cathodic reactions [38].
%) was calculated using Eq.5. From the results, no significant shift was noted on Ecorr values. If the shift in (Ecorr) values for the inhibited solution is more than 85mV with reference to the Ecorr of the blank, then the inhibitor could be classified as anodic or cathodic type and if the shift is below 85mV, the inhibitor might be regarded as a mixed type inhibitor [36, 37]. This indicates that AMLE acts as mixed-type inhibitor. The results also showed that Icorr values decreased with increase in concentration of the extract. This indicates that the extract molecules were adsorbed on the mild steel surface and thus inhibits the corrosion of the metal in H2SO4 solution. Further inspection of the results revealed that values of βa and βc change slightly in the presence of the extract, signifying that the extract control both anodic and cathodic reactions [38].
Figure 5: Polarization curves for mild steel corrosion in 2M H2SO4 in absence and presence of different concentrations of AMLE at 303K.
Table 5: Polarization parameters for mild steel corrosion in 2M H2SO4 in absence and presence of different concentrations of AMLE at 303K.
|
Extract conc.(g/l) |
Ecorr(mV) |
Icorr(µA/cm2) |
βa(mV/dec) |
βc(mV/dec) |
η% |
|
Blank |
-500 |
101 |
400 |
520 |
- |
|
0.1 |
-489 |
60 |
420 |
620 |
41 |
|
0.5 |
-530 |
41 |
370 |
660 |
59 |
Electrochemical impedance measurements
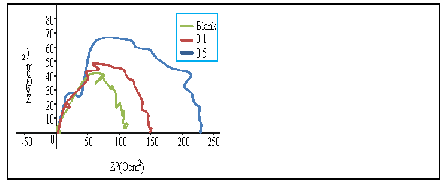
The impedance spectra of mild steel in 2M H2SO4 solution in the absence and presence of different concentrations of AMLE at 303K are presented in the form of Nyquist diagrams in Fig.6. The Nyquist diagrams show a semicircular appearance, signifying a charge transfer process mainly controls the corrosion of the mild steel. It is also observed from Figure 6 that the diameter of the Nyquist plots increased on addition of different concentrations of the plant extract. This indicates adsorption of the extract molecules on the metal surface [15]. The calculated impedance parameters are shown in Table 6. As seen in Table 6, the double layer capacitance (Cdl) values decreased while the charge transfer (Rct) values increased with the extract concentrations. The decrease in Cdl values could be attributed to the decrease in the local dielectric constant and/or an increase in the thickness of the electrical double layer. Moreover, the decreased in Cdl values with increased values of Rct and the inhibition efficiency might be due to the gradual replacement of water molecules by the adsorption of the extract molecules on the metal surface, which led to formation of protective layer on the metal surface. [39,40]. The %IE values increased with the extract concentrations and maximum  was achieved at 0.5g/l of the extract (Table 6).These results suggest that the extract molecules were adsorbed on the metal surface. Similar observations have been reported [41-43].
was achieved at 0.5g/l of the extract (Table 6).These results suggest that the extract molecules were adsorbed on the metal surface. Similar observations have been reported [41-43].
Figure 6: Nyquist plot of mild steel in 2M H2SO4 in absence and presence of different concentrations of AML extract.
Table 6: Electrochemical Impedance Parameters of Mild Steel corrosion in 2M H2SO4 in the absence and presence of different concentrations of AMLE 303K.
|
Extract conc.(g/l) |
Rp(Rct) Ωcm2 |
fmax |
Cdl\ (µF/cm-2) |
%IE |
|
Blank |
118.59 |
41.83 |
3.213 |
- |
|
0.1 |
251.27 |
61.69 |
1.027 |
52.80 |
|
0.5 |
308.38 |
114.59 |
0.604 |
61.54 |
Surface Morphological Investigations
FT-IR
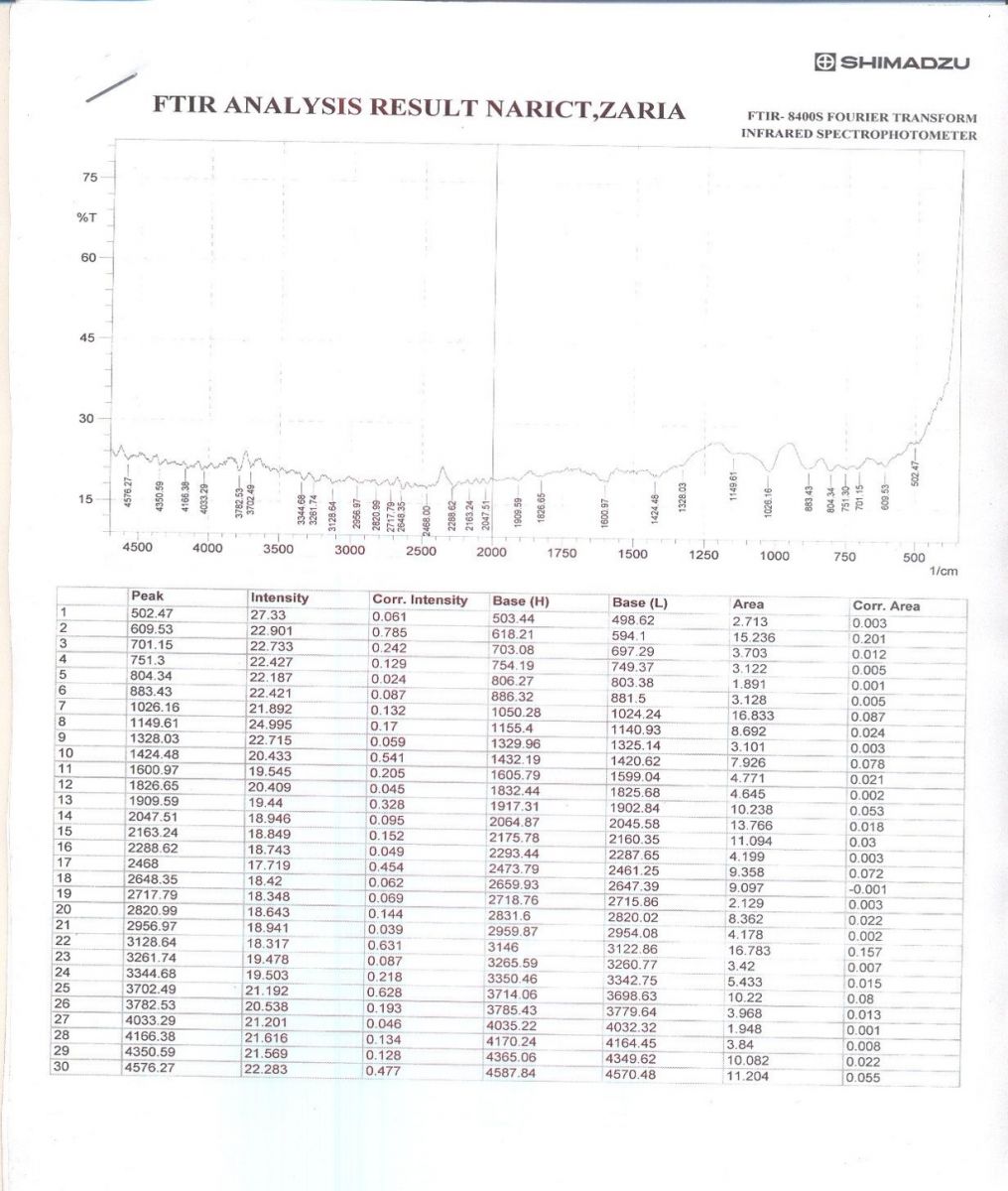
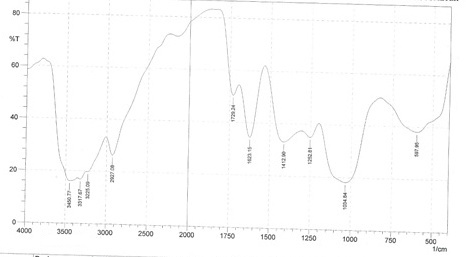
The FTIR spectra have been used to analyze the film formed on the mild steel surface. The spectra of pure AMLE and the corrosion product is shown in Figures 6a and b.
The peak numbers obtained from the spectra of the pure AMLE and the corrosion products as well as the assigned functional groups are shown in Table 6. It is seen from the result that some peaks have shifted to higher frequency region and some peaks which were present in Fig 6a disappeared in Figure 6b. Also, additional peaks were noticed in the region of 1252.81cm-1, 1623.15cm-1 and 1729.24cm-1in Fig. 6b.The change in peak values revealed that there is an interaction between the extract and the mild steel surface which leads to inhibition of the corrosion. This is in line with the previous reports [44-47].
Figure 6 a: FTIR spectrum of pure AMLE.
Figure 6 b: FTIR spectrum of the corrosion product on mild steel in the presence of AMLE in 2M H2SO4 solution.
Table 6: IR frequencies of AMLE and corrosion product on mild steel in H2SO4 solution with AMLE
|
Pure AML extract |
Corrosion product on mild steel surface |
||
|
Frequency (cm-1) |
assigned functional groups |
Frequency (cm-1) |
assigned functional groups |
|
3347.67 |
N-H stretch of amines |
3364.68 |
N-H stretch of amines |
|
3225.09 |
O-H stretch of alcohols and phenols |
3251.74 |
O-H stretch of alcohols and phenols |
|
2927.97 |
C-H stretch of alkanes |
2958.08 |
C-H stretch of alkanes |
|
2820.00 |
C-H stretch of alkanes |
2853.9 |
C-H stretch of alkanes |
|
2163.24 – 2288.62 |
C-N |
2163.24 – 2288.62 |
Peaks disappeared |
|
1826.65 |
C=O stretch |
1849.24 |
C=O stretch of carbonyl |
|
1412.90 |
C=C stretch |
1434.48 |
C=C stretch |
|
1149.61 |
C-O stretch of ethers |
1157.72 |
C-O stretch of ethers |
|
1026.16 |
C-N stretch |
1034.84 |
C-N stretch of amine |
|
609.47 |
C-H stretch |
697.95 |
C-H stretch |
Scanning Electron Microscopic analysis
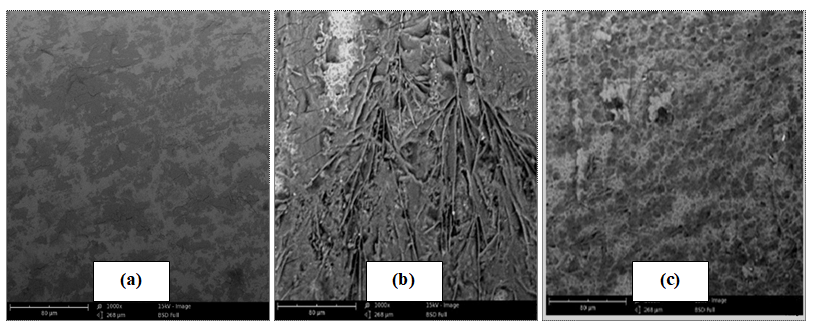
SEM micrographs of mild steel coupons before and after 18hrs immersion in 2M H2SO4 solution without and with AMLE are shown in Figure 7a - c. The surface of mild steel coupon before immersion in test solution (fig 7a) appeared clean and smooth. Figure 7b shows that the surface of mild steel was rough and corroded in 2M H2SO4 solution, whereas in the presence of the extract the coupon surface was comparatively smooth as observed in Figure 7c. This signifies that the presence of the extract retards the acid attack on the mild steel surface. This is in agreement with previous study [18, 30, 33, 48] who attributed the decrease in the rate of corrosion on metal surface to the adsorbed extract components which formed a protective barrier between the metal surface and the acid medium.
Figure 7: SEM micrographs of mild steel surface (a) before immersion, (b) after 18hrs immersion in H2SO4, (c) after 18hrs of immersion in H2SO4 with 0.5g/l AMLE at 303K
CONCLUSION
Acanthus montanus leaves extract was found to inhibit the corrosion of mild steel in 2M H2SO4 solution to reasonable extent. Inhibition efficiency values increased with increasing concentration of the extract but decreased with rise in temperature. Polarization results showed that AMLE acted as a mixed-type inhibitor. EIS studies show that Rct increased with increasing extract concentration while the double layer capacitance reduced. Additionally, higher values of Rct were obtained in the presence of the extract signifying adsorption of the extract molecules on the mild steel surface. The mechanism of physical adsorption has been proposed, and a first order type of reaction was obtained from the kinetic treatment of the experimental data. The adsorption mechanism obeyed Langmuir adsorption isotherms model at all concentrations and temperature ranges. Protective film formation against acid attack was confirmed by FT-IR and SEM techniques.
REFERENCES
- Eduok UM, Etim UJ, Akpakpan AE, Umoren SA. (2012). Corrosion inhibition and adsorption behaviour of Cocosnucifera L coir dust for mild steel in 1M HCl: Synergistic effect of iodide ions. Int J Adv Sci Tech Res. 2(1):338–360.
- Marko C, Fidelis C. (2016). Recent Natural Corrosion Inhibitors for Mild Steel: An overview. J Chem: 1 -7.
- Oguzie EE, Enenebeaku CK, Akalezi CO, Okoro SC, Ayuk AA, Ejike EN. (2010). Adsorption and corrosion inhibiting effect of Dacryodisedulis extract low-carbon-steel corrosion in acidic media. Journal of Colloid and Interface Sci. 349:283-292.
- Harikumar S, Karthikeyan S. (2012). Inhibition of mild steel corrosion in hydrocholoric acid solution by cloxacillin drug. J Mater Environ Sci. 3(5):925-934.
- Cookey GA, Tambari BL, Iboroma DS. (2018). Evaluation of the corrosion inhibition potentials of green-tip forest lily (Clivia nobilis)leaves extract on mild steel in acid media. J Appl Sci Envir Manag. 22(1): 90-94.
- Vera R, Figueredo F, Díaz-Gómez A, Molinari A. (2018). Evaluation of Fuji Apple peels extract as a corrosion inhibitor for Carbon Steel in a saline medium. Int J Electrochem Sci. 13:4139-4159.
- Abiola OK, Tobun Y. (2010). Cocosnucifera L. water as green corrosion inhibitor for acid corrosion of aluminium in HCl solution. Chinese Chem Let. 21:1449-1452.
- Udom GA, Cookey GA, Abia AA. (2018). Corrosion inhibition and Adsorption Characteristics of Myrianthusaboreus leaves extract on Copper in Sulphuric acid solution. Chem Sci Int J. 23(3):1-13.
- Iroha NB, Akaranta O, James AO. (2012). Corrosion inhibition of mild steel in acid media by Red peanut skin extract-furfural resin. Adv Appl Sci Res. 3(6):3593-3598.
- Olasehinde EF, Olusegun ST, Adesina AS, Omogbelin SA, Momoh-Yahayah H. (2013). Inhibitory action of Nicotianatobacum extracts on the corrosion of mild steel in HCl: Adsorption and Thermodynamics Study. Nat Sci. 11(1):83-90.
- Chebouat E, Dadamoussa B, Gherraf N, Gouamid M, Allaou M, Cherite A, et al. (2013). Inhibition of mild steel corrosion in 1N HCl medium by acid extract of Ephedra alata. Int J Electrochem Sci. 8:12147-12153.
- Leelavathi S, Rajalakshmi R. (2013). Dodonaeaviscosa (L.) Leaves extract as acid corrosion inhibitor for mild steel-A Green approach. J Mat Envir Sci. 4(5):625-638.
- Murthy ZVP, Vijayaragavan K. (2014). Mild steel corrosion inhibition by acid extract of leaves of Hibiscus sabdariffa as green corrosion inhibitor and sorption behavior. Green Chem Let Rev. 7(3):209-219.
- Nwaugbo, C.M. & James, A.O. (2014). The corrosion inhibition of mild steel in sulphuric acid solution by flavonoid (catchin) separated from Nypafruticans wurmb leaves extract. Sci J Chem. 2(4):27-32.
- Muthukrishnan P, Jeyaprabha B, Prakash P. (2014). Mild steel corrosion inhibition by aqueous extract of Hyptissuaveolens leaves. Int J Ind Chem. 5:5.
- Fadare OO, Okoronkwo AE, Olasehinde EF. (2016). Assessment of anticorrosion potentials of extract of Fiscus asperifolia-Miq (Moraceace) on mild steel in acidic medium. African Journal of Pure and Applied Chem. 10(1):8- 22.
- Alaneme KK, Olusegun SJ, Adelowo OT. (2016).Corrosion inhibition and adsorption mechanism studies of Hunteria umbellateseed husk extracts on mild steel in acidic solutions. Alexandria Eng J.55(1):673-681.
- Ameh PO, Kolo AM, Ahmed A, Ajanaku IK. (2017). Electrochemical study of the corrosion inhibition of Delonixregia for mild steel in sulphuric acid medium. J Indust Envir Chem. 1(1):15-21.
- Adam MK, Odola PO, Aji IS. (2016). Assessment of Honey as corrosion inhibitor on mild steel. Seminar Series. 7:78-83.
- Elham A, Mohamed MR, Seham SE, Magda E, Rabab M, Becnel JJ, Khan I. (2012). Potent insecticidal Secondary Metabolites from the medicinal plant Acanthus montanus. Records Nat Prod. 6(3):301-305.
- Orlu EE, Obulor A. (2014). Investigation on the effect of aqueous Leaf Extract of Acanthus montanus on spermatogenesis in Swiss mice. J Pharm Biol Sci. 9(3):44-49.
- Igwe A, Eleazu C. (2018). Effect of Processing on the Biochemical content of Acanthus montanus (Nee) T. Anderson (Acanthaceae) leaves. Food Sci Nutr. 6:388-394.
- Udom IG, Cookey GA, Abia AA. (2017). The Effect of Acanthus montanus Leaves Extract on Corrosion of Aluminium in Hydrochloric Acid Medium. Current J Appl Sci Technol. 25(2):1-11.
- Jonathan MN, Efiok JA, Adeyemi OO. (2015). Inhibition and Adso-kinetic studies of Gmelina arborea fruit extract on corrosion of armour steel plate in hydrochloric acid. Int J Sci Eng Res. 6(9):701-717.
- Satapathy AK, Gunasekaran G, Sahoo SC, Kumar A, Rodrigues PV. (2009). Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution. Corr Sci. 51:2848–285.
- Umoren SA, Obot IB, Ebenso EE. (2008a). Corrosion Inhibition of aluminium using exudate gum from Pachylobus edulis in the presence of Halide ions in HCl. E- J Chem. 5(2) 355-364.
- Okafor PC, Ebiekpe VE, Azike CF, Egbung GE, Brisibe EA, Ebenso EE. (2012). Inhibitory action of Artemsia annua extract and artemisinin on the corrosion of mild steel in H2SO4 solution. Int J Corrosion:1-8.
- Njoku VO, Oguzie EE, Obi C, Ayuk AA. (2014). Baphianitida leaves extract as a green corrosion inhibitor for the corrosion of mild steel in acidic media. Adv Chem. 2014:1-10.
- Shukla SK, Ebenso EE. (2011). Corrosion Inhibition, Adsorption Behavior and Thermodynamic Properties of Streptomycin on Mild Steel in Hydrochloric acid Medium. Int J Electrochem Sci. 6:3277-3291.
- Fouda AS, Mohamed AE, Khalid MA. (2016). Trigonella stellate extract as corrosion inhibitor for Copper in 1M Nitric acid solution. J Chem Pharm Res. 8(2):86-98.
- Abiola OK, Abdulraman OCA, Suleiman M. (2016). Eco-friendly corrosion inhibitors: Effect of delonixregia extract on corrosion and kinetics of corrosion process of aluminum alloy 2S in HCl solution. Afr Corrosion J. 2(2):11-16.
- Mohd N, Ishak AS. (2015). Thermodynamic Study of Corrosion Inhibition of Mild Steel in Corrosive Medium by Piper nigrumExtract. Ind J Sci Technol. 8:17.
- Cheng G, MA L Q, Gui XH, Liu JT, Wang YT. (2013). Study on Kinetic Modeling for Fine Coal Flotation. IntJ Coal Preparat Utilization. 33:12–25.
- Al-Mhyawi RS. (2014). Corrosion inhibition of Aluminum in 0.5 M HCl by Garlic aqueous extract. Oriental J Chem. 30(2):541-552.
- Okewale AO, Adesina OA. (2020). Kinetics and Thermodynamic Study of corrosion inhibition of Mild Steel in 1.5M HClmedium using Cocoa leaf extract as inhibitor. J Appl Sci Environ Manage. 24(1):37-47.
- Namrata C, Dileep KY, Vinad KS, Qurashi MA. (2015). A comparative study of leaves extracts for corrosion inhibitors effect on aluminium alloy in alkaline medium. Ain Shams Eng J. 8(2):1-10.
- Anadebe VC, Onukwuli OD, Okafor NA. (2018). Optimization and Electrochemical study on the Control of Mild Steel Corrosion in Hydrochloric acid solution with Bitter Kola leaf extract as Inhibitor. S Afr J Chem. 71:51–61.
- Fouda AS, Elmorsi MA, Abou-Elmagd BS. (2017). Adsorption and inhibitive properties of methanol extract of EeuphorbiaHeterophylla for the corrosion of copper in 0.5M nitric acid solutions. Polish J Chem Technol. 19(1):95-103.
- Zarrouk1 A, Zarrok H, Salghi R, Touir R, Hammouti B, Benchat N, et al. (2013). Electrochemical impedance spectroscopy, weight loss and quantumchemical study of new pyridazine derivative as inhibitor corrosion of copper in nitric acid. J Chem Pharm Res. 5(12):1482-1491.
- Fouda AS, Shalabi K, Idress AA. (2015). Ceratoniasiiqua extract as green corrosion inhibitor for copper and brass in nitric acid solutions. Green Chem Let Rev. 8(3-4):17-29.
- Shah AM, Rahim AA, Homid SA, Yahya S. (2013). Green inhibitors for copper corrosion by Mangrove Tannin. Int J Electrochem Sci. 8:2140–2153.
- Patel KK, Vashi RT. (2015). Azardirachtaindica extract as corrosion inhibitor for Copper in Nitric acid medium. Res J Chem Sci. 5(11):59-66.
- Uchenna LE, Nnamdi CI, Kate OC, Okechukwu DO. (2019). Corrosion Inhibition of Mild Steel by Citrus Sinensis (Orange) leaves Extract in HCl/H2SO4 acid medium. Int J Adv Res Chem Sci. 6(7):1-9.
- Kavitha N, Manjula P, Anandhakumar N. (2014). Synergistic effect of C. Papaya Leaves Extract-Zn2+ in Corrosion Inhibition of Mild Steel in Aqueous Medium. Res J Chem Sci. 4(8):88-93.
- Alaneme KK, Olusegun SJ. (2012). Corrosion Inhibition Performance of Lignin Extract of Sun Flower (TithoniaDiversifolia) on Medium Carbon Low Alloy Steel immersed in H2SO4 Solution. Leonardo J Sci. 20:59-70.
- Weiqing Z, Shuguang J, Kai W, Menglu M. (2015). Thermogravimetric Dynamics and FTIR Analysis on Oxidation Properties of Low-Rank Coal at Low and Moderate Temperatures. Int J Coal Preparation Utilization. 35(1)
- Al-Fakih AM, Aziz M, Sirat HM. (2015). Turmeric and ginger as green inhibitors of mild steel corrosion in acidic medium. J Mater Environ Sci. 6(5):1480-1487.
- Ameh PO. (2018). Electrochemical and computational study of gum exudates from Canarium schweinfurthii as green corrosion inhibitor for mild steel in HCl solution. J Taibah Univ Sci. 12(6):783 –795.
 Abstract
Abstract  PDF
PDF
.PNG)